How Do Underserved Communities Prefer To Learn About Clinical Research?
By Stephanie Loomer, CISCRP

The importance of clinical trial research in developing treatments and advancing healthcare is widely acknowledged. In 2019 alone, 46,391 study volunteers contributed to clinical trials that resulted in the approval of 48 novel drugs. Of the demographic subpopulations represented, 9 percent of study volunteers were black/African American, 9 percent were Asian, and 18 percent were Hispanic, highlighting a lack of representation of underserved populations.1 Recently, the FDA recommended broadening eligibility criteria to enhance diversity in clinical trials and therefore better reflect the patients who will be using a drug once it is approved.2
Pharmaceutical companies have begun working to address these disparities in clinical trials. For example, companies such as Sanofi and Eli Lilly have partnered with patient organizations and medical associations to better connect with underserved communities.3 When identifying study sites, both companies include geographic locations with diverse populations in their searches. Furthermore, Eli Lilly requires that at least two sites be in diverse locations for larger research studies.3 These are a few of the ways that companies can engage a more inclusive group of study volunteers and better understand how the drug will impact the broader target patient population upon approval.
While these efforts are critical steps in the right direction, it is equally important to look at the bigger picture and better understand core participation motivators and barriers from the underrepresented patient’s perspective in order to better enable access to and increase their participation in clinical trials. Every two years, the Center for Information and Study on Clinical Research Participation (CISCRP) conducts a global online study to gather data from the public and patients on their perceptions and experiences with clinical research. Responses from over 12,450 individuals were collected, with 6 percent and 10 percent self-identifying as black and Asian, respectively, and 13 percent of Hispanic origin. Here are some highlights and learnings from the perspective of underserved communities that can be applied to promoting and increasing diversity and inclusion in clinical research studies.
Acknowledging The Importance Of Clinical Research
There is wide acknowledgement of the importance of clinical research across races and ethnicities as nearly all (98 percent) consider clinical research studies “somewhat/very important” to the discovery and development of new medicines. The greatest benefits of clinical research participation included helping to advance science and the treatment of the patient’s disease/condition (26 percent) and the possibility of improving or saving the lives of others with the same condition (21 percent), indicating that study volunteers recognize how they can help themselves and others through research. Despite these positive perceptions, few respondents had seen or heard about a clinical trial recently. Specifically, Asian (53 percent) and Hispanic (52 percent) individuals were the least likely out of any group to recall recently learning about a research opportunity. Issues of trust and concerns about risk also exist; 33 percent of black individuals reported having little or no trust in pharmaceutical companies and 28 percent of Asian individuals have never participated in a clinical trial because of concerns associated with risk.
Where Do People Look For Clinical Trials?
Targeting areas where individuals look for research opportunities is an essential step in reaching underserved populations. A significant gap remains between where individuals would prefer to learn about clinical research and where they currently learn about opportunities.
As shown in Table 1, many would begin their search for a clinical trial by asking their healthcare provider, yet groups reported various other sources for identifying research opportunities. Over half of black individuals (52 percent) would likely use an online clinical trial registry, Hispanic individuals (52 percent) would likely ask their primary care physician, and Asian individuals would use an internet search engine like Google (42 percent). Recommendations from family members were also particularly important to Asian, Hispanic, and black individuals (20 percent, 15 percent and 14 percent, respectively).
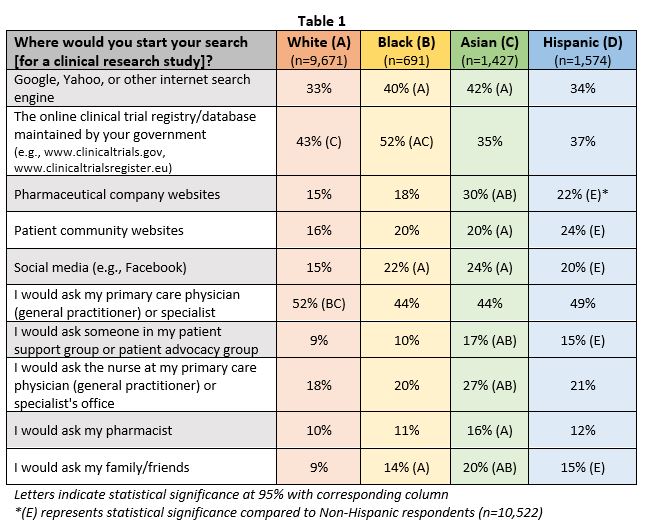
Healthcare providers remain an untapped channel to increase awareness of and participation in clinical trials among underserved populations. The majority find it very important for their healthcare provider to be aware of ongoing clinical trials in their local communities, yet less than 25 percent of Hispanic and black individuals were informed of a clinical trial by their doctor or nurse. Furthermore, 66 percent of black individuals report being more willing to participate if their own doctor was conducting the trial, far more than any other race and ethnicity.
Services, Education, Patient Connections Can Help Increase Participation
Identifying and providing supportive services that matter most to these underserved communities is essential to improving their access to clinical trials. Black (46 percent) and Hispanic (44 percent) individuals report that concierge services during their participation are very important. Concierge services may include arranging for transportation assistance to/from study clinic visits or providing meals that accommodate a patient’s diet. Over half of black individuals (56 percent) also identified monetary compensation as an important consideration, particularly in cases where a study volunteer may miss work to attend a study clinic visit. Incorporating these supportive operational elements into clinical trials can positively impact study enrollment and retention.
Healthcare offices and online formats were highlighted as critical points of engagement for increasing diversity and inclusion in clinical trials. These spaces, and others, also present opportunities for patient education and community connection. As seen in Table 2, many agree that doctors are the best and most preferred way to understand more about and identify clinical trials. However, minority groups identified additional avenues where people could best learn about the clinical research process. Receiving educational information from pharmaceutical companies was reported by 26 percent of black and Asian individuals. Twenty one percent of each minority group also reported that an educational program in school or college was another way to learn about clinical research.
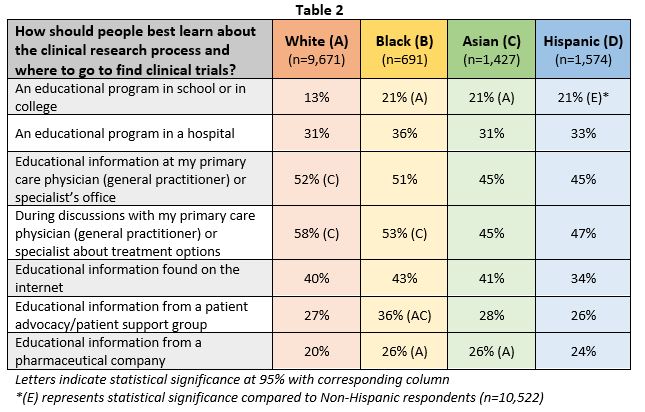
In addition, patient advocacy or support groups were identified as resources for learning more about clinical research, especially by black individuals (36 percent). Connecting with other patients in an online format was also highlighted as valuable among underserved populations. For example, Hispanic (83 percent), black (80 percent) and Asian (75 percent) individuals were “somewhat/very interested” in discussing and getting advice on participating in a clinical research study with peers in an online patient community.
Organizations can utilize these channels for patient education and connection. Providing information about clinical research can give underserved communities the tools to make informed decisions when choosing to participate. Allowing for patients to connect with one another can build trust within communities and help groups learn more about their conditions.
Conclusion
Organizations can promote diversity and inclusion in clinical trials by leveraging established trusted healthcare provider and patient relationships in various ways – starting with informing healthcare providers about new clinical trial opportunities in their communities to share with their patients. It is also important to recognize where patients currently begin their search for trial opportunities. Focusing recruitment efforts specifically where underserved groups are currently seeking trials presents another opportunity to engage this audience.
Underserved populations want to better understand their condition and connect with others in their community. A common thread among black, Asian, and Hispanic populations is the power of technology in learning more about their condition and the study and connecting with others throughout the research process. These are just some initial ways organizations can address current disparities and increase the involvement of diverse, representative patients in future clinical trials.
References:
- U.S. Food and Drug Administration. (2019). Drug Trials Snapshots Summary Report. https://www.fda.gov/media/135337/download.
- U.S. Food and Drug Administration. (2019). Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. Draft Guidance issued June.
- Myshko, D. (2018). Addressing diversity in clinical trials. PharmaVOICE. Retrieved April 2, 2020, from https://www.pharmavoice.com/article/2018-03-diversity/.
About The Author:
Stephanie Loomer is a project manager for the Research Services department at the Center for Information & Study on Clinical Research Participation (CISCRP). She received a bachelor’s degree in anthropology from Plymouth State University and a master’s degree in medical anthropology and cross-cultural practices from Boston University School of Medicine. She started at CISCRP in 2019 after having worked in research focused on substance use disorders.
