How Do You Determine If RWE Is A Good Fit For Your Org? Ask (And Answer) The Right Questions
By Ivanna Rosendal, senior director at Ascendis Pharma and podcast host of Transformation in Trials

Real world evidence (RWE) is being heralded as a way to enrich our clinical data and lower the barriers to reimbursement. It also carries the potential of reducing the patient burden and lowering the cost of clinical data acquisition. But before getting to the point where we can reap the promises of RWE, we face a number of challenges as an industry. If we can overcome them, we can build a path to RWE — and arrive at the benefits it will bring.
Direction And Collaboration Are Key Challenges To Leveraging RWE
Beginning an RWE initiative is a question of how we understand what we are trying to achieve and organizing toward that aim. In May, I attended an RWE session at the ISPOR conference in Boston where I got the chance to ask a room full of medical and market access participants what they found most challenging:
- Making cross-functional collaboration work, or
- Finding the necessary data sources for RWE?
The majority of the room voted for A. And even the people studying rare diseases, for which data is perpetually hard to find, agreed that establishing a constructive, cross-functional team is even harder. Building that kind of collaboration in a larger company, with both global and local functions, just multiplies the complexity. The question remains: How can we deal with some of these challenges and build a road toward RWE?
Understanding Why RWE Is A Fit For Your Organization
Like many other terms that are en vogue, RWE has a slightly different meaning, and reason why it should be used, depending on who you ask. There may not be a definition applicable across the entire industry, but you can agree on what RWE is to your company and how it can bring benefits to your specific situation.
To help facilitate this alignment, I have developed the following RWE strategy canvas:

Figure 1: RWE Strategy Canvas.
This canvas is best used in an iterative fashion, with more information being added to the individual quadrants as you learn more.
Considering The Data Recipients
As a starting point though, I would suggest that you think about the recipients of the data you would like to collect. All evidence is an argument of sorts, where you are attempting to influence the perception of someone toward the argument you are making. Making a good argument depends on who you are attempting to persuade. To influence a medical regulatory agency, you may need to focus on the efficacy and safety of your drug. If you are aiming to influence a reimbursement agency, you may want to focus on data that will argue that your drug is worth investing in from a societal perspective.
Once you understand who the recipients of your analysis will be, it is worth considering their concerns and how they prefer to receive and consume data. Will you need to provide data that they will want to analyze on their own? Should it be brought forth in collaboration with a key opinion leader (KOL) or other neutral party to ensure credibility? Is it data that needs to be published in an influential magazine to have weight? These considerations will influence the design of your RWE plan.
Understanding Your Data Gaps
Understanding who you want to influence with your data allows you to keep your aim true when you start considering your situation and determining where you have a gap in data that needs to be addressed. Knowing what we would like to claim and what we can claim based on our clinical data is key to understanding our data gaps. This is where the interdisciplinary collaboration between market access, commercial, and clinical teams comes into play. We need to understand the market conditions for competitors, market preference, and patient experience deficiencies of standard of care to understand where current data falls short. It could be related to adherence, quality of life, drug reimbursement positioning or other matters. Identifying the market opportunities and mapping our current data to them will reveal where we may want to collect additional data.
When the gaps are clear, the question is whether collecting evidence from the real world is the right tool to address this gap. Here I would recommend brainstorming all possible ways that this data gap can be addressed — including preclinical modelling, investigator-initiated trials, competitor intelligence, registry studies, publications, and other available data sources. Having that list of every possible way that the data can be acquired will help identify the narrow path of getting the right data quality, with the least amount of effort and cost.
Mapping these opportunities on a simple graph like the below can help focus the efforts on the ideas with highest data quality and easiest acquisition. To complete this exercise, you do not need to have perfect knowledge of all the ideas. The purpose is to focus your investigation on the ideas with the highest potential. When you investigate them in detail, you may learn that your assumptions about, for example, how easy they are to access were wrong. And that is OK. Then you simply continue to the next idea.
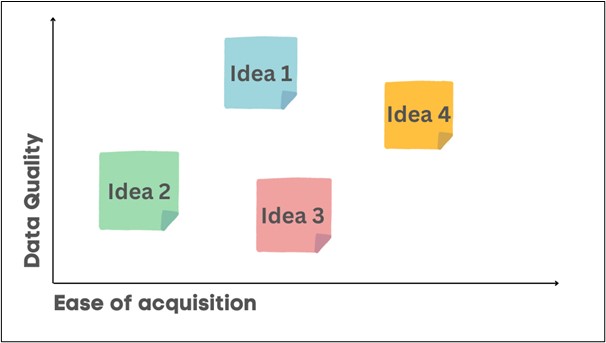
Figure 2: Prioritizing data acquisition opportunities.
Thinking Through Your RWE Initiative Delivery
Once you understand your target audience and your data gaps, it is time to start considering how you are going to bring your RWE initiative to life.
Depending on your data gap, you will need to consider who needs to be involved internally to ensure sufficient expertise to this matter. Typically, you will need to involve the following functions:
- Commercial
- Medical
- Clinical
- Market Access
- Digital
- Regulatory
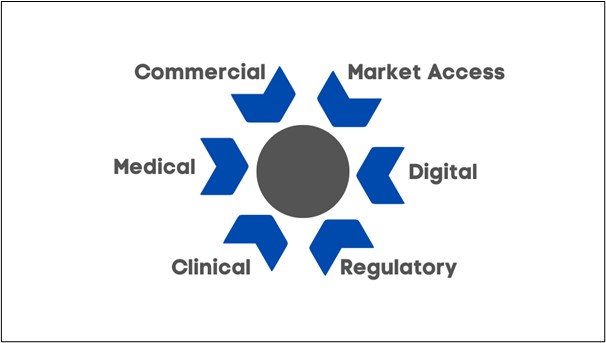
Figure 3: Functions needed to ensure RWE initiatives are a success.
All these functions contribute to understanding the RWE initiative and ensuring that the argument for your target audience holds water. It is challenging to make these functions work together though, since they are anchored in different disciplines and speak different professional languages.
I would suggest including a facilitator for the RWE initiative, who ensures that there is a cadence and rhythm for the interaction between these stakeholders. Determining rules for productive conversations and decision rights for specific meetings also can help ensure progress. Investing in making these stakeholders feel like a team is worth the effort. This can include creating a team charter and investing the time to do an offsite meeting to ensure alignment on what the initiative is about and how it will be delivered — and the expectations to each individual contributor.
The complexity of this collaboration can balloon in larger organizations, where there may be local and global RWE initiatives that can easily become divorced from one another. The organization needs to decide whether to pursue a global RWE strategy or if the local entities are “set free” to pursue local agendas. But even in these cases, it’s important to ensure that knowledge is shared between the local and global level to identify synergies.
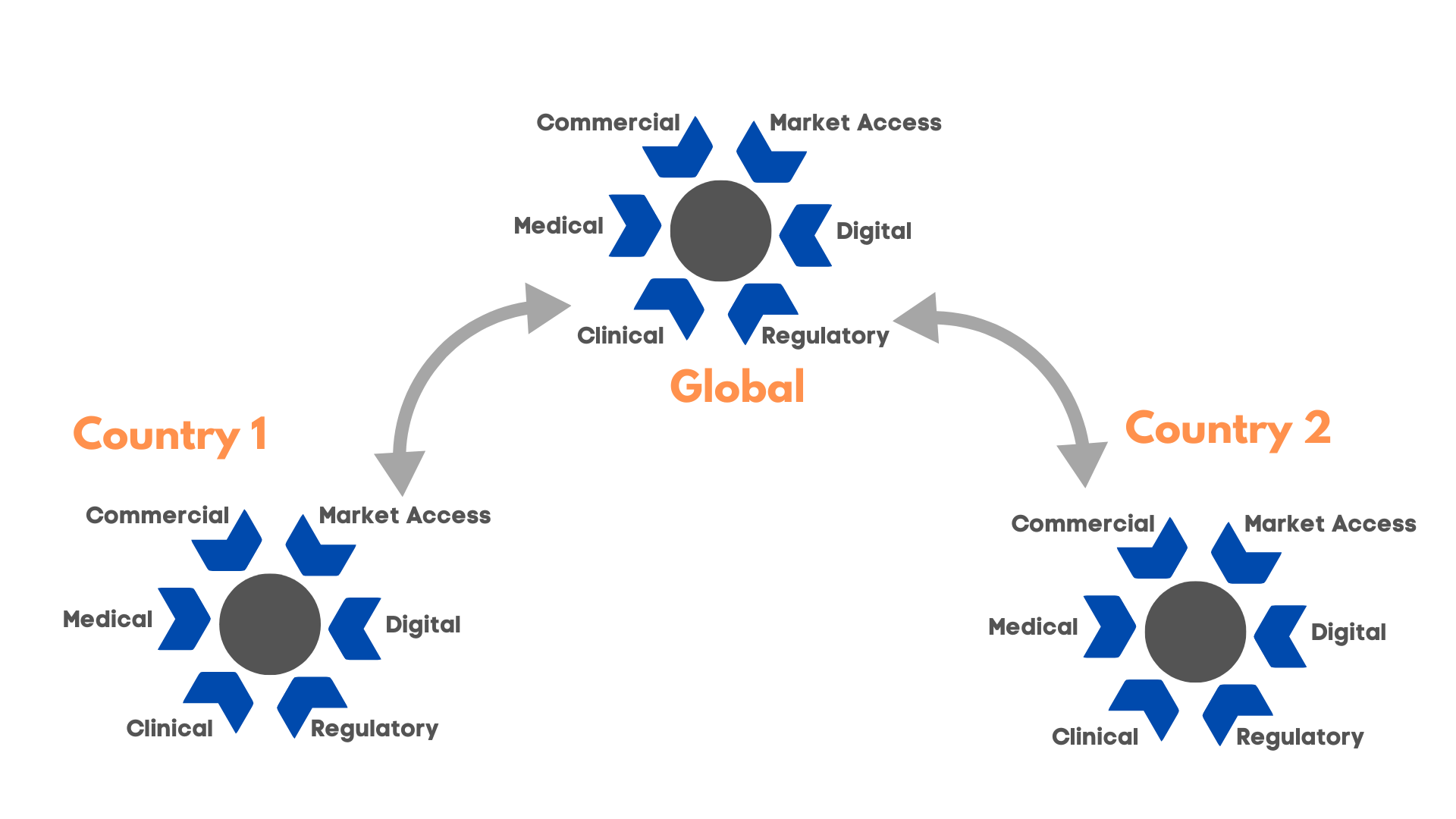
Figure 4: Global and local RWE initiatives
Getting the necessary functions in the same room and ensuring they can work as a team leads to the next question: Should this be fulfilled using internal competencies, or is there a need to engage a partner?
Depending on your RWE strategy, you may or may not have competencies in-house. If the data gap you are addressing is likely to be repeated for other drugs in your pipeline, it’s worth investing in internal competencies to acquire and process this type of data. This also goes for investment in internal platforms for data modelling.
Checking Yourself: What Are Your Constraints?
After you have considered your data audience, selected gaps to focus on, and put together the team with the right competencies, it’s time to check yourself. Creating the perfect canvas for an unconstrained RWE initiative is a risk. Consider your key constraints.
Key constraints could be market dynamics — if a competitor is launching a product at the same time as you and has better data in the area where you have a gap. This constraint may mean that even though you would like to build capabilities in house in the future, you may need to engage with a partner in the present to achieve speed of execution.
Another key constraint may be funds. You could find yourself in a situation where you do not have the funds for your preferred initiative. Checking yourself against this reality may mean that you reconsider the data sources or the scope of the initiative you have planned. Or, select another initiative altogether.
Organizational capacity also can be a constraint. Making RWE work requires commitment from a broad set of organizational stakeholders. If the organization is constrained for time due to other strategic priorities, you may not be able to get the right people at the table. This means that you may need to realistically gauge which people you can engage and select an initiative that can suffice with the skillsets available.
Iterating Toward A Clear, Doable RWE Initiative
Checking your constraints may mean that you take another round with the RWE strategy canvas. But that is the point. Taking a couple of iterations on the canvas will ensure that you land on a RWE strategy that is both focused and doable, so that you can tell that data story to the audience you want to influence.
Building your RWE initiative means thinking through what RWE means to you. The RWE canvas can be a helpful tool in this aspect, making sure that you consider your audience, your data gaps, and how you can get the right skillsets at the table. Trying out the canvas in your organization could be the first step undertaking the path to RWE.
About The Author:
 Ivanna M. Rosendal is the senior director of IT in Ascendis Pharma. She is a behavioral economist and has held technology leadership positions in life sciences for the past 10 years. She hosts a podcast about innovation in clinical trials called Transformation in Trials and writes about femtech wearables for TechTruster. She is also the author of the book Maneuvering Monday, that is currently available as a podcast. Reach out to Ivanna via LinkedIn.
Ivanna M. Rosendal is the senior director of IT in Ascendis Pharma. She is a behavioral economist and has held technology leadership positions in life sciences for the past 10 years. She hosts a podcast about innovation in clinical trials called Transformation in Trials and writes about femtech wearables for TechTruster. She is also the author of the book Maneuvering Monday, that is currently available as a podcast. Reach out to Ivanna via LinkedIn.
