How Doubly Labeled Water Can Improve Data In Metabolic Disease Research
By Hari Mix, CEO, and Erica Svendahl, research scientist, Calorify
Metabolic diseases and conditions related to obesity and diabetes have gained attention as healthcare costs from chronic diseases continue to grow. Currently, nearly 42% of Americans suffer from obesity. By 2035, that number is set to reach nearly 58% of the population, a rise of nearly 70 million individuals, and the global cost of obesity is expected to surpass $4.3 trillion, equivalent to 3% of global GDP.
While the root of these omnipresent diseases is complex and multifactorial (including obesogenic environments, psycho-social factors, and genetic variants), the fundamental cause is an imbalance of calories consumed and expended1, resulting in mitochondrial and metabolic dysfunction that can present itself in many ways, from obesity and cancer to Alzheimer’s and schizophrenia.2 The concept of energy balance underlies every response to metabolic disease. From diets and exercise regimens to drugs and surgical interventions, every solution works (or fails) by reducing caloric intake and/or increasing caloric burn.
While this concept is simple to understand, almost no clinical trials or research measure it (only 11,714 measurements are hosted in the global database3). Doubly labeled water is the only scientifically proven method to determine both sides of the energy balance equation: total energy expenditure and total energy intake.
The Doubly Labeled Water Solution
The doubly labeled water (DLW) method is the only proven technique for measuring human energy expenditure in any environment (i.e., under free-living conditions).4 Discovered in 19495 and used in humans since 19826, this form of indirect calorimetry uses labeled hydrogen (deuterium) and oxygen (oxygen-18) to measure CO2 production.
“The fundamental basis of the DLW method is that oxygen turnover in the body is dominated by the flow of water through the body as well as inspired oxygen and expired carbon dioxide. The turnover of body hydrogen, however, is dominated only by the flow of water through the body. Consequently, the difference between the turnovers of oxygen and hydrogen provides a measure of the excess efflux of oxygen that is equivalent to the production of carbon dioxide.”6 Expired carbon dioxide is the product of cellular respiration and is equivalent to total energy expenditure (Figure 1).
The testing process for subjects is simple, consisting of a single sip of DLW and the collection of three urine samples over the course of a week — all from home, with no lab visits required. This kind of testing is invaluable in clinical trials because the absence of required lab visits helps capture highly contextual data. At-home testing does not disrupt schedules and is passive while participants live their lives, yielding the most representative metabolic data.
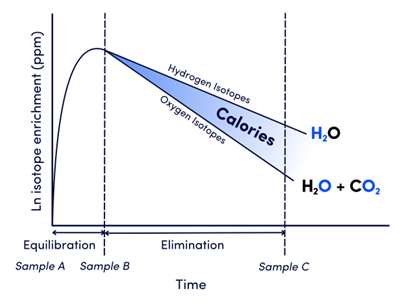
Figure 1. The course of enrichment for oxygen and hydrogen isotopes throughout the course of a DLW test to determine CO2 production (equivalent to calories burned).
In addition to providing the only free-living measurement of energy expenditure, DLW is the gold standard method for determining total energy intake when combined with daily weigh-ins.7 This is essential because people are notoriously bad at tracking their energy intake. It is easy to be off by hundreds of calories, even with a food scale, and nutritional labels are not always completely accurate.
Finally, DLW also offers the most accurate at-home body composition test via deuterium dilution. This method has comparable accuracy and precision to a DXA scan.8 At-home body composition tests can change the clinical trial landscape in three major ways: reducing expensive equipment and trial sites associated with DXA scans, eliminating calibration of different instruments, and making trial recruitment more inclusive and thus faster because there are fewer equipment-driven body size constraints. In all, the overall trial cost is reduced because fewer personnel and trial sites are necessary, and the recruitment process can move faster with fewer participant restrictions.
The Importance Of Measurements In Obesity Clinical Trials
A 2022 Obesity Medicine Association Clinical Practice Statement emphasized the benefit of assessing both energy expenditure and body composition in patients with pre-obesity or obesity.9 This metabolic data serves as an impactful tool as clinical trials continue to utilize precision medicine. DLW has already been regarded as a missed opportunity in recent trials evaluating dietary interventions and their success based on various -omics mechanisms.10 Metabolism is highly individual and changes throughout weight loss (via metabolic adaptation and loss of lean body mass), making DLW extremely complementary to studies of a similar nature.11,12 Additionally, as weight loss drugs (including GLP-1s) gain prevalence, DLW can evaluate these drugs from both sides of the energy balance equation, in addition to evaluating body composition changes.
DLW adds another level of accessibility as well because body composition measurements via deuterium dilution do not have weight or body size limits. Currently, DXA scans often have weight limits of around 400 lbs., thus excluding some patients.
In time, this extensive metabolic data set from across various trials will be incredibly valuable. Combined with genetic, bloodwork, and other existing health data, scientists will leverage its proprietary metabolic data set and AI to drive solutions to metabolic diseases from diabetes to Alzheimer’s, heart disease, and cancer.
Obesity Clinical Trials Should Leverage DLW
Obesity and other metabolic diseases are a tremendous and still-growing problem in the U.S. and around the world. Energy imbalance is at the root of metabolic disease, yet it isn’t being measured. The DLW method offers gold-standard assessments of total energy expenditure, energy intake, and body composition for clinical and research evaluations of metabolic disease and related treatments. DLW has the power to change the clinical research landscape by reducing temporal and monetary costs associated with trial recruitment and testing.
References:
- WHO. 2024. Obesity.
- Sergi et al. 2019. Mitochondrial (Dys)function and Insulin Resistance: From Pathophysiological Molecular Mechanisms to the Impact of Diet. Frontiers in Physiology.
- AEA. 2024. DLW Database.
- Westerterp. 2017. Doubly labeled water assessment of energy expenditure: principle, practice, and promise. European Journal of Applied Physiology.
- Lifson et al. 1949. The fate of utilized molecular oxygen and the source of the oxygen of respiratory carbon dioxide, studied with the aid of heavy oxygen. Journal of Biological Chemistry.
- Speakman. 1998. The history and theory of the doubly labeled water technique. American Journal of Clinical Nutrition.
- de Jonge et al. 2007. Validation study of energy expenditure and intake during calorie restriction using doubly labeled water and changes in body composition. American Journal of Clinical Nutrition.
- Melanson et al. 2018. Validation of the doubly labeled water method using off-axis integrated cavity output spectroscopy and isotope ratio mass spectrometry. American Journal of Physiology Endocrinology and Metabolism.
- Burridge et al. 2022. Obesity history, physical exam, laboratory, body composition, and energy expenditure: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obesity Pillars.
- Qi et al. 2023. Toward Precision Weight-Loss Dietary Interventions: Findings from the POUNDS Lost Trial. Nutrients.
- Flanagan et al. 2024. No evidence for metabolic adaptation during exercise-related energy expenditure. iScience.
- Pontzer. 2018. Energy Constraint as a Novel Mechanism Linking Exercise and Health. Physiology.
 About The Authors:
About The Authors:
Hari Mix, Ph.D., is Calorify’s founder and CEO. He completed his graduate and undergraduate degrees at Stanford University. He studied isotopes and ran track, both of which led him to start Calorify, commercializing DLW testing. His expertise in making precise, isotopic measurements combined with his passion for metabolism has made him the perfect candidate to drive DLW commercialization forward.
 Erica Svendahl is a research scientist at Calorify. She has won many awards for her research prior to joining Calorify. She brings a wealth of lab expertise that has helped drive Calorify’s lab to provide high-quality data, fast.
Erica Svendahl is a research scientist at Calorify. She has won many awards for her research prior to joining Calorify. She brings a wealth of lab expertise that has helped drive Calorify’s lab to provide high-quality data, fast.
