How To Accelerate Digital Clinical Transformation Through A Holistic Approach
By Krishnan Rajagopalan, PhD, president, Life Sciences – Sourcing Advisory and Consultancy Services

Leveraging technology for competitive advantage and sustained benefits has been a core lever for many industries over the last few decades, but particularly so in the last few years. In the life sciences industry, the use of digital platforms and technologies and unprecedented collaboration between companies, governments, and regulators led to the development of COVID-19 vaccines in less than a year, compared to the seven to eight years of a traditional development cycle.1 Yet there is a broader belief in the industry that it can be even more agile in developing and delivering needed and safer medicines faster by leveraging digital transformation.
Life science companies are pursuing digital initiatives across many processes, but the following seven areas are expected to deliver maximum benefits due to digital transformation initiatives:
- Efficient, real-time data access
- Sponsor systems and site selection analysis
- Technology-enabled patient systems
- Scientific analysis for program design
- Digitally-enabled patient recruitment and retention
- Risk-based and centralized monitoring
- Digital technologies to automate human processes.
While newer digital, AI, mobile, and cloud technologies are becoming available, companies are struggling to design, implement, and realize the comprehensive digital transformation benefits. Even though 90% of companies have started some type of digital transformation initiatives, only a third of the expected ROI/revenue benefits, on average, have been realized.2 In the successful cases, comprehensive planning and a holistic, integrated approach across strategy, process, technology, data, people, and other areas created the transformational value.
Leveraging A Digital Clinical Transformation Maturity Framework
Based on LS-SACS's experience working with life sciences clients and digital product vendors, interviews with trial sites, and the articulation of digital transformation levers by The Open Group,3 we are providing a holistic approach for the design and implementation of a successful digital clinical transformation program in this article. Companies can first use a systematic digital clinical transformation maturity assessment (DCTMa) to help understand their status in the context of “best in class” methods and trends within life sciences and other industries, identify gaps, and develop a holistic digital transformation roadmap.
Ten key enterprise capabilities for successful digital clinical transformations are discussed below, using an illustrative output of a DCTMa.
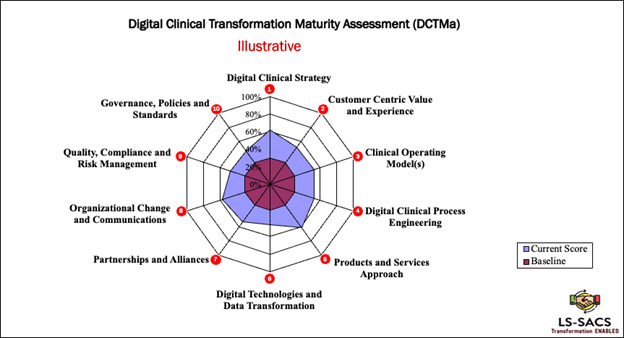
1. Digital Clinical Strategy: Life sciences companies must create a digital clinical strategy that includes the following key steps:
- Develop a strategic plan and road map complete with milestones; desired outcomes and metrics; required capabilities across people, process, and technology; and planned investments. The plan should cover both short-term (e.g., pilot projects/priority areas of digital clinical transformation maturity) and long-term initiatives aligned with the company’s digital strategy.
- Identify and establish a dedicated and empowered digital clinical leadership team with the right skills and experience that can drive and manage digital clinical transformation from strategy through implementation in a sustained manner.
- Communicate the strategic plan and performance metrics to all the departments, functions, strategic external partners, and internal team members that are part of the digital transformation programs (e.g., clinical operations, medical affairs, and commercial).
2. Customer Centric Value and Experience (CCVE): A digital strategy should clearly identify customer segments and sub-segments (e.g., patients, investigators, regulators) and their perceived value experience as inputs to the digital strategic objectives. Given the difficulties in patient recruitment and retention, as well as the importance of patient centricity and diversity in clinical trials, the voice of the patient must be integrated with the digital transformation strategy. Typical analysis must include:
- Value Segmentation: Covers specific value propositions for each segment, potential digital services and products for each segment, and methods to monitor and refine perceived value and customer satisfaction.
- Positioning and Marketing: Analyzes the extent of recognition of brand, products and services, multiple channels (e.g., patient and investigator portals with optimized content), and the ability to compare the company’s methods with those of competitors.
- Personalization and Retention: Includes clear understanding of the value drivers behind each of the customer segments’ loyalty and satisfaction across channels and the ability to refine and customize rapidly to meet the expectations.
- Expansion of Customer Base/Cross-selling: Measures the company’s proactiveness in identifying and implementing expansion opportunities – e.g., extension of new digital approaches for patient recruitment and retention.
- Rescue and Win-back: Analyzes the turnover rates per patient segment, reasons for turnover/dissatisfaction, and planned approaches to reach out and win these patients back.
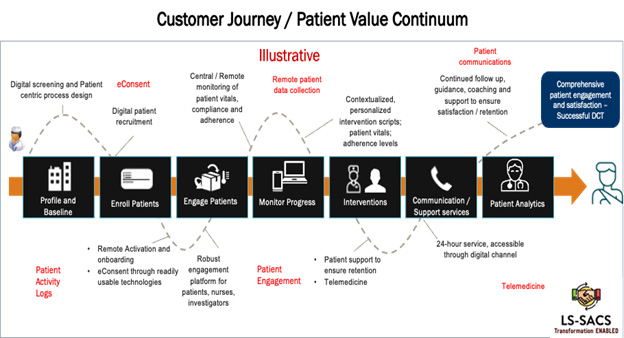
3. Clinical Operating Models: The digital clinical leadership Team, working closely with clinical operations, IT, commercial and other functional teams, must evaluate each potential operating model (e.g., DCTs, hybrid, traditional, RBM) that can deliver the clinical strategic objectives. Each model requires its own processes/workflows; digital, mobility and AI technologies; and staff support and partnerships that have the expertise to support such models. For example, a full DCT would require end-to-end technology capabilities for multichannel digital patient recruitment. They would also need the infrastructure, internal staff, and external partnerships for at-home nurse visits in all trial geographies and remote follow-up. Proactive planning for each of these needs along with the allocation of appropriate budgets to support them is critical to the success of digital transformation.
4. Digital Clinical Process Reengineering/Optimization: Just as business transformation utilizes business process reengineering (BPR), digital transformation requires digital clinical process reengineering (DCPR) that leverages digital technologies to give organizations more options to redefine how existing tasks will be performed in a digital trial environment. For example, future processes should cover redefined roles and responsibilities of both internal staff and external teams as well as data and information flows, triggers, and flags for real-time updates using workflows. The DCPR objective is to use redefined clinical trial models advantageously and alter the pharma company’s competitive positioning to create true “digital clinical disruption.”4
5. Products and Services Digitalization Approach: Digitalization involves leveraging data and analytics, as well as digital technologies such as social, mobile, cloud, AI, and IoT to optimize and automate manual business process activities to create new products and services focused on the outcomes that all customers and patients expect.
Strategically driven digitalization can help pharma companies improve patient access to trials and follow-up care, reduce wait times, and deliver more personalized and effective treatments. In addition, it can help sponsors and investigators track patient outcomes and identify areas for improvement in real time. To ensure the best ROI and improved patient outcomes through the digital products and services, both customer experience and digital excellence need to be aligned.
6. Digital Technologies and Data Transformation: Mobile, cloud, Big Data, AI, automation, AR-VR, and wearable technologies help in the engagement of patients, get data from additional sources for real-time analytics and decision making for “active” patient management, and improve patient satisfaction and retention. Evaluation, identification, and leverage of the “right” technology products and collaborations that align with a company’s digital clinical strategy and operating models is crucial for an effective transformation. Some pharma companies feel their site selection, patient data capture/analytics, and automation platforms are the least mature capabilities in clinical operations.5 Yet acquiring the digital technologies alone isn’t enough. In many cases, DCT technologies are considered as an encumbrance rather than an enabler for site personnel. Therefore, it’s crucial to consider these tools’ improvements to data access, ability to identify and measure the correct digital endpoints, and ease of use among patients and investigators through effective change management and training.
Choosing the most appropriate digital technologies and integrating them with other data sources (clinical, safety, regulatory, RWE/HEOR, etc.) can enable digital clinical technology infrastructure to provide the most accurate data. This can allow clinical trial leaders to develop insights and make real-time decisions. One of the most important benefits of such an integrated data and analytics is the ability to measure the health data of clinical trial participants continuously and unobtrusively during their daily lives, called data continuomics, rather than at a specific point in time.6
7. Partnerships and Alliances: True digital clinical transformation requires a closely connected ecosystem of both internal and external partners. Some of the key partners include7outsourced service providers, data and information providers, technology providers and systems integrators, patient support providers, investigator sites, academic institutions, and pharmacies, among others.
It is critical that a clear partnership strategy and communication plan be developed and monitored for digital transformation to be successful.
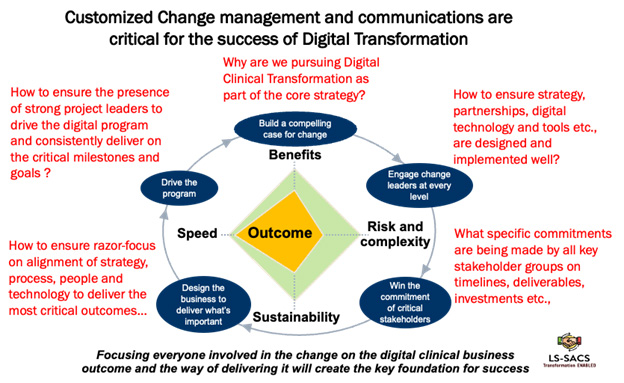
8. Organizational Change and Communications: Clinical trial organizations typically have teams working across functional areas in many locations, which can complicate operations in companies transitioning through a mix of traditional and hybrid trials toward a fully digital clinical trials operating model. A targeted and customized organizational change management (OCM) and communication approach and detailed plan that also covers sites and patients becomes a critical component for a successful digital clinical transformation initiative.
A successful change strategy needs to be customized to the objectives of digital clinical transformation and leverages accountable senior leaders (“change leaders”) with expertise in both clinical operations/key therapeutic areas and digital clinical tools/processes. These leaders actively engage the cross-functional teams and drive the change needs for key processes at the right time. They can help overcome many of the organizational and cultural barriers by educating not only internal teams but also investigators and patients about the vision and objectives of the digital transformation initiative.
9. Quality, Compliance, and Risk Management: Given that digital clinical transformation requires modified processes, new technologies, and expanded stakeholders, pharma companies need to refine their overall enterprise risk management (ERM) framework and tools. Some of these stakeholders have less structure and oversight compared to traditional sites and, hence, create an opportunity for more variability in the data. For example, telemedicine, virtual visits, and home health services may present increased risk among the multiple DCT modalities. The breadth of contributing risk types suggests the need for a more expansive risk assessment and mitigation process.7
ERM framework and policies generally cover four areas: strategy, operations, reputation, and technology. Each of these factors and their sub-factors need to be refined in the context of digital clinical transformation and operating modalities of DCT and quantifiable measures and metrics developed for proactive tracking and monitoring.
The clinical operations part of the ERM framework can mostly be managed well due to the increase in adoption of the key features of both quality by design (QbD) and risk-based quality management (RBQM) that can allow the tracking and monitoring of potential risks generated in a digital clinical trial. QbD ensures that quality is built into the trial process from the beginning, and RBQM focuses on risk management and quality improvement through a framework that allows study teams to focus on the most critical data. For example, RBQM and central monitoring tools (e.g., KRIs) can enable monitoring entities to identify those that are not performing as needed and should be part of the overall ERM framework to ensure tracking and adherence.
10. Governance, Policies, and Standards: Life sciences organizations have realized the potential of digital transformation through positive learnings from the COVID-19 response. The shift across the healthcare and life sciences industries continuum to patient centricity (“customer centric value and experience”), increased use of digital products and solutions/services, collaborations that extend beyond the traditional partners, and potential risks of “siloed” and tactical digital initiatives makes an overarching and empowered digital transformation governance critical for success.
The diagram below depicts a digital transformation governance framework and the key pillars.
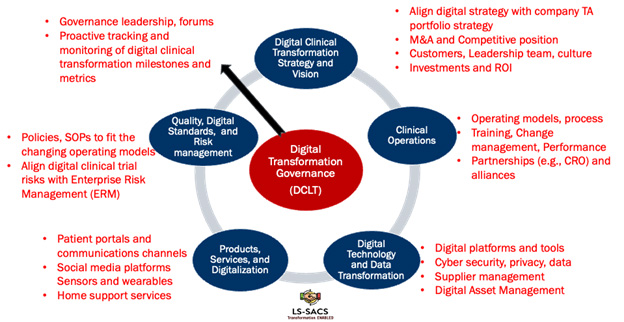
The life sciences industry is trying to catch up with the digital leaders across other industries. While efforts have increased over the past few years within life sciences companies, they still trail other industries by a factor of two or three in most dimensions.9 Life sciences companies need to stop “doing digital” with tactical and siloed digital initiatives and instead “be digital” through not only making digital transformation a strategic imperative for the organization but also ensuring they have the overarching governance and leadership/talent to implement the strategy and plans successfully. Adopting an agile approach to integrating digital and analytics with their traditional focus on patient centricity and delivering innovative therapies will go a long way to making life sciences organizations more competitive and delivering greater benefits to patients and shareholders.
References:
- https://www.umms.org/coronavirus/covid-vaccine/testing#:~:text=Although%20the%20first%20vaccines%20were,ensuring%20their%20>BR?safety%20and%20effectiveness.
- https://www.mckinsey.com/capabilities/mckinsey-digital/our-insights/rewired-to-outcompete
- www.opengroup.org
- https://blog.ventanaresearch.com/digital-process-reengineering-drives-business-change
- https://scrip.pharmaintelligence.informa.com/SC147650/Pharmas-Clinical-Ops-Digital-Maturity-Patient-Experience-Tools-Lag
- The Future of Drug Trials is Better Data and Continuous Monitoring, HBR, May 02, 2019
- https://www.appliedclinicaltrialsonline.com/view/survey-results-gcp-quality-and-risks-in-decentralized-clinical-trials
- https://cyntegrity.com/synergy-of-qbd-rbm-and-rbqm-in-trials/
- Top 10 observations in Life Sciences digital and analytics. McKinsey Survey Results, November 2022.
About the Author:
 Krishnan Rajagopalan, PhD is the president of Life Sciences – Sourcing Advisory and Consultancy Services and brings 30 years’ experience in management consulting and advisory, strategic sourcing and outsourcing and global services delivery to Fortune 100 clients. He has advised small, medium, and large life sciences clients and digital/SaaS product companies on growth strategy, organizational change and communications, operational model design and implementation, strategic and sourcing and outsourcing (CRO, BPO, FSP and IT services). Krishnan also delivered full-service CRO, CDM, pharmacovigilance/safety, biostatistics/programming, MW, regulatory affairs, commercial services and IT/digital to more than 100+ life sciences companies through Fortune 500 global services providers from India, China, Argentina, multiple European countries, and the U.S. He can be reached via email, by phone at +1 908 380 3343, and at https://www.linkedin.com/in/dr-krishnan-rajagopalan-bab7ba16/
Krishnan Rajagopalan, PhD is the president of Life Sciences – Sourcing Advisory and Consultancy Services and brings 30 years’ experience in management consulting and advisory, strategic sourcing and outsourcing and global services delivery to Fortune 100 clients. He has advised small, medium, and large life sciences clients and digital/SaaS product companies on growth strategy, organizational change and communications, operational model design and implementation, strategic and sourcing and outsourcing (CRO, BPO, FSP and IT services). Krishnan also delivered full-service CRO, CDM, pharmacovigilance/safety, biostatistics/programming, MW, regulatory affairs, commercial services and IT/digital to more than 100+ life sciences companies through Fortune 500 global services providers from India, China, Argentina, multiple European countries, and the U.S. He can be reached via email, by phone at +1 908 380 3343, and at https://www.linkedin.com/in/dr-krishnan-rajagopalan-bab7ba16/
