Hybrid Decentralized Trials: Using Technology To Create More Patient-Centric Studies
By Dan Milam, VP, Global Engagement, Society for Clinical Research Sites (SCRS)
While it is not a new trend, the spotlight on digital innovation has grown brighter over the last year, with executives from all sides of the clinical research industry seeking technology solutions to improve the clinical trial experience for sites and patients alike. It was a recurring theme at this year’s Society for Clinical Research Sites (SCRS) Global Site Solutions Summit, with several sessions that addressed technological advancement and attendee feedback requesting more discussion on the topic at future Summits.
Ultimately, the goal of clinical trial technology is to increase patients’ awareness of and access to clinical trials. Patient recruitment is a top struggle, but the more convenient and accessible we are able to make trial participation, the more likely and inspired a potential patient will be to participate.
A panel of patients speaking to SCRS Global Summit attendees shared that although they had all participated in clinical trials, none were currently in one. They cited lack of access as a primary reason – too much travel, inconvenient office hours, too many in-person visits, and other stringent requirements that made it difficult to fit the trial into their lives and ultimately led them to not participate in another clinical trial or to drop out of the initial trial. This patient feedback made the need to increase access to clinical trials as a care option abundantly clear.
A patient-centric trial design is a powerful method to solve enrollment problems. Implementing suitable technology is the easiest and most reliable pathway to aligning patients with clinical trials that fit their lifestyles and needs.
SCRS conducts an annual Site Landscape Survey to gather data from global clinical research sites on trends in the industry. Receiving a record number of responses, this year’s survey took a deep look into technology trends in the industry. Seventy-five percent of site respondents had been in business for 11+ years and thus have experience tracking these trends.
Patient-centricity can be enhanced through the use of technology services. In response to the industry’s trend toward patient-centric efforts, sponsors and CROs have begun partnering with sites to take a hybrid approach to conducting clinical trials by participating in decentralized clinical trials (DCTs) — also referred to as virtual trials – in which some study visits are conducted remotely. Remote visits take place in the comfort of the patient’s home and can be completed by using mobile technology that both sites and patients can access from a designated mobile phone or computer; some providers even offer the option to download an app directly onto the patient’s personal device.
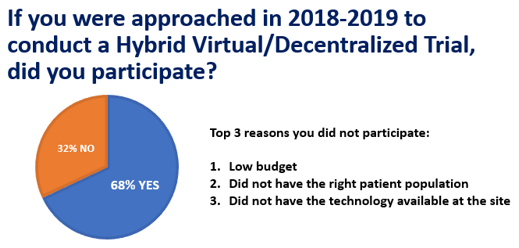
Of the 15 percent of survey respondents who noted they had been approached by a sponsor or CRO to conduct a hybrid DCT, 68 percent said yes. The 32 percent of sites that declined indicated that supporting a variety of technologies based on multiple studies and sponsors was one of their top barriers to participation. More than half of the respondents said they would do whatever is required to prepare to conduct a DCT, including actively upgrading their knowledge of technology and upgrading the systems used on-site.
Technology can be used to enhance both a site’s and a sponsor’s/CRO’s ability to better meet patients’ needs. Through adopting services such as direct-to-patient shipping and remote nursing services, we are seeing more and more clinical trial activities moving from being conducted at the site to being conducted at the patient’s home. Further, the use of mobile technologies has made it possible for patients that previously were not able to participate in clinical trials, such as elderly or immobile populations, those who live far from the nearest site, and individuals without the financial means to travel, to receive access to valuable and life-saving treatments.
Despite the need to prioritize the patient experience and the reality that technology is becoming essential to increasing patient-centricity, many sites are understandably concerned about the impact of digital innovation on security, quality, and usability. More often than not, when a site bypasses technology that could improve processes and create better access for its patients, it is a direct response to these concerns. These concerns are important and it is vital to find resolutions.
It is never more important to move forward thoughtfully than when implementing something new into the clinical trial space. Solutions providers are hard at work to ensure that they are not only delivering a quality product, but also creating a clear pathway to adoption and implementation. Sites should be proactive and take the lead in this industry shift by creating standard operating procedures that support the adoption of new technology while also mitigating the delays inherent to learning new processes. Doing so will position sites at the forefront of the digital innovation conversation, rather than waiting for a system to be developed and designed by sponsors/CROs that sites are then required to adhere to.
While clinical research sites will no doubt always play an integral and irreplaceable role in the conduct of safe and effective clinical trials, these changes demonstrate that the future of medicine does not exist solely within the four walls of the site — it extends to the patient’s home as well. This is the time for sites to redefine their role in this ongoing conversation. Sites can ensure their prominent place in the clinical trial landscape of the future and remain at the forefront of innovation by embracing technology as adoption continues at a rapid pace across the clinical research industry.
Technology alone cannot change the face of clinical research, but the right technologies, adopted with forethought and strategy, can. The question is not if but when each site will be asked to participate in a DCT. As sponsors and CROs begin designing more and more protocols that include a virtual component, sites must increase their knowledge of and access to technology that supports engaging in hybrid DCTs.
About The Author:
 Dan Milam is VP of Global Engagement at the Society for Clinical Research Sites (SCRS), whose mission is to unify the voice of the global clinical research site community for greater site sustainability. With over 20 years of experience working in the pharmaceutical, CRO, and site sectors, Milam has gained a wide perspective and understanding of the needs of the industry as a whole that aids in facilitating and building strong alliances. His focus is on expanding SCRS’ global footprint and the inclusion of all industry stakeholders in SCRS initiatives with the goal to foster sustainability for clinical research sites.
Dan Milam is VP of Global Engagement at the Society for Clinical Research Sites (SCRS), whose mission is to unify the voice of the global clinical research site community for greater site sustainability. With over 20 years of experience working in the pharmaceutical, CRO, and site sectors, Milam has gained a wide perspective and understanding of the needs of the industry as a whole that aids in facilitating and building strong alliances. His focus is on expanding SCRS’ global footprint and the inclusion of all industry stakeholders in SCRS initiatives with the goal to foster sustainability for clinical research sites.
