I AM INCLUDED: 8 Best Practices For Sites To Drive More Inclusive Clinical Trials
By Jeanne M. Regnante, Elizabeth George, Shanda Cooper, Belinda Paulicin, and Maimah Karmo, I AM INCLUDED Expert Alliance

Historically, clinical trial recruitment efforts have often prioritized convenience and accessibility for certain groups, inadvertently marginalizing underserved populations. To get to the nirvana of accessibility for all, we must infuse clinical trial sites with soul — led by patients, patient organizations, caregivers, providers, site staff, local leaders, lay people, and influencers. To that end, Tigerlily Foundation partnered with Labcorp to design a transformational, modernized, multi-stakeholder framework for authentic and successful clinical trial site practices to enable more inclusive clinical trials. This holistic framework — I AM INCLUDED — not only enhances inclusivity but also strengthens trust and engagement, ultimately leading to improved health outcomes for all.
What Is The I AM INCLUDED Framework?
In-depth interviews and two listening summits with leaders from leading historically Black colleges and universities (HBCUs), health systems in the U.S., leaders of patient organizations, community leaders, and patients — known as the I AM INCLUDED Expert Alliance — yielded 115 best practices organized into nine themes: patient and caregiver engagement actions, upstream actions, site workplace and operations actions, study execution design actions, trial launch and accrual actions, end of trial actions. community engagement actions, institutional actions, and ecosystem and policy actions.
The primary principle behind I AM INCLUDED is that clinical trial sites must lead. We must ask them for their perspective on behalf of the population that they serve. We must give sites sustained budget support, commit to partnering authentically with them and the communities they serve, and be accountable to the site and those it serves. That means sponsors, CROs, patient organizations, and community-based organizations must be aligned with the site to support accessibility, drive early engagement, foster communication, and build trust with patients. When they are all aligned, this will demonstrate accountability and dedication to understanding inclusivity, enrollment, and disparities in care where people live.
Who Can Use the I AM INCLUDED Framework?
This framework can be used successfully across all types of sites, including academic sites, community sites, and physician practice sites, as well as across therapeutic areas. It is also intended to be used by all types of clinical trial sites, community-based academic or health systems, life sciences organizations, manufacturers, CROs, patient advocacy organization leaders, community and faith-based organizations, patients, and peer or clinical navigators.
In fact, once I AM INCLUDED became available for public use, those who downloaded it were surveyed about their intentions. Confirming the validity of its usefulness, 60% of respondents reported that they were somewhat likely or extremely likely to act on the best practice recommendations from I AM INCLUDED, and 67% reported that they were extremely likely to recommend I AM INCLUDED to a friend or colleague.
A core recommendation of the I AM INCLUDED framework is enlisting community clinicians and including them as sub-investigators to work hand in hand with the community for trusted engagement. This will help to extend the reach of clinical research by adding a vast pool of potential participants, thereby reducing recruitment delays, and increasing the likelihood that the trial population will resemble the demographic of patients who will eventually take the drug/medical product when it reaches the market.
How Does I AM INCLUDED Align With Industry Diversity Guidance?
I AM INCLUDED’s patient and community engagement practices uphold much of the FDA’s draft diversity action plan (DAP) guidance.1 FDA’s suggested practices include but are not limited to “implementing sustained community engagement (e.g., through community advisory boards and navigators, community health workers, patient advocacy groups, local healthcare providers, community organizations, etc.); providing cultural competency and proficiency training for clinical investigators and research staff; improving study participant awareness and knowledge of the clinical study; providing transportation assistance; providing dependent care; allowing flexible hours for study visits; [and reimbursing] patients for costs incurred.”
Patient and community-based organization insights gathered from the I AM INCLUDED listening summits, social media, and the survey emphasized these FDA recommendations. The path forward is clear: we must continue to work authentically with patients and communities, valuing their voices and lived experiences, to ensure inclusive, equitable, and lifesaving access to clinical trials for all. Interest among those surveyed was consistent with sentiments expressed by representatives from FDA and NIH, clinical investigators, pharmaceutical companies, community-based organizations, and others who were asked about best practices for engaging underrepresented populations in clinical trials. This publication2 concluded that community engagement is the most effective strategy for recruiting and retaining underrepresented groups.
How Can Sites (And Others) Get Started Quickly?
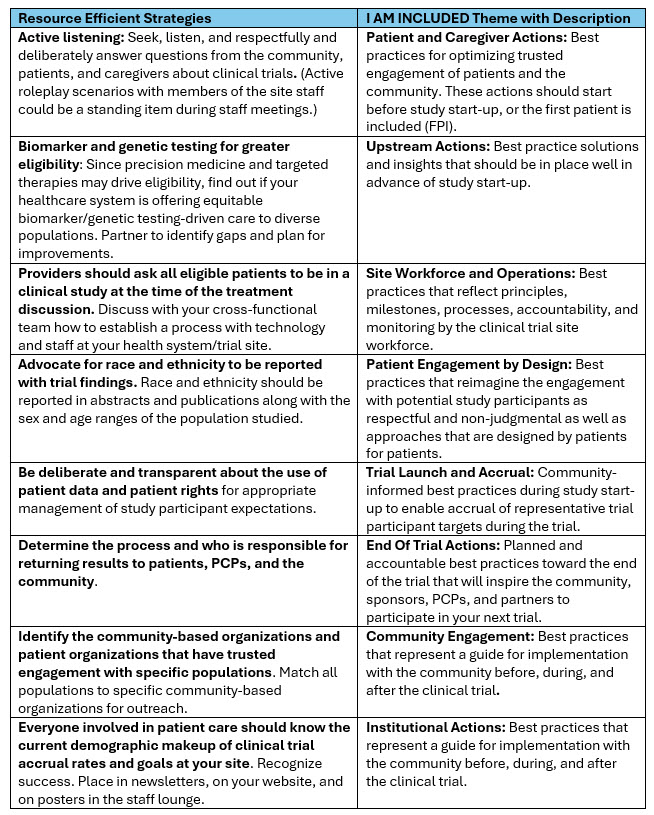
Noting the variable levels of resources available to different sites, the I AM INCLUDED Alliance identified eight low-resource strategies (as defined by cancer centers whose leaders reported successful accrual of racial and ethnic minority populations in clinical trials)3 that may yield incremental, quality, and efficient improvements. They recommended the following actionable practices by theme to quickly help site staff and partners get some “quick wins.” The I AM INCLUDED Expert Alliance concluded that, in their experience, these strategies do not take a lot of time, staffing, or funding to implement. Authors used a definition of low resource strategies previously reported by 8 cancer centers whose leaders reported successful accrual of racial and ethnic minority populations in clinical trials.3
TABLE 1. I AM INCLUDED Expert-Informed Resource Efficient Strategies by Theme
Tigerlily and Labcorp are honored to bring this framework, with expanded stakeholder and expert feedback, forward to support site leaders and research centers that advance inclusive clinical research and bring new medicines and devices to market that will benefit all populations with preventable and chronic disease.
References:
- Food and Drug Administration. Diversity action plans to improve enrollment of participants from underrepresented populations in clinical studies draft guidance for industry, (https://fda.gov); (Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies | FDA)
- Kelsey MD, Patrick-Lake B, Abdulai R, et al. Inclusion and diversity in clinical trials: actionable steps to drive lasting change. Contemp Clin Trials. 2022;116:106740. doi:10.1016/j.cct.2022.106740
- Regnante JM, Richie N, Fashoyin-Aje L, Hall LL, Highsmith Q, et al. Operational strategies in US cancer centers of excellence that support the successful accrual of racial and ethnic minorities in clinical trials, Contemporary Clinical Trials Communications. (2020), Volume 17,2020,100532, ISSN 2451-8654,(https://www.sciencedirect.com/science/article/pii/S2451865420300168) ; https://doi.org/10.1016/j.conctc.2020.100532
- Tigerlily Foundation. Recommendations for clinical trial site leaders: an actionable framework for inclusive clinical trials, November 2023, I Am Included – Clinical Trials | Tiger Trials | Tigerlily Foundation
About the I AM INCLUDED Expert Alliance:
Shonte Drakeford, MSN, CRNP, AGNP-C, Metastatic Breast Cancer Patient Advocate
Kim F. Rhoads, MD, MS, MPH, FACS, Associate Professor, Epidemiology & Biostatistics, Affiliate Philip R. Lee Institute for Health Policy Studies; Associate Director, Community Engagement, Helen Diller Family Comprehensive Cancer Center, UCSF School of Medicine; Founder, Umoja Health Partners
Angelo D. Moore, PhD, MSN, RN, NE-BC Executive Director, Center of Excellence for Integrative Health Disparities & Equity Research. North Carolina Agricultural and Technical State University, CIHDER, Greensboro, NC.
Isagani M. Chico, MD, Vice President and Global Head, Oncology Therapeutic Expertise, Fortrea
Rajbir Singh, MBBS, Executive Director, Precision Medicine & Health Equity Trials Design Clinical and Translational Research Center, Center for Women's Health Research; Assistant Professor, Internal Medicine, Meharry Medical College
Kedar Kirtane, MD, Assistant Member Physician Director for Engagement of Special Populations for Clinical Trials, Department of Head and Neck-Endocrine Oncology, Moffitt Cancer Center
Evelyn González, MA, Senior Director, Office of Community Outreach, Fox Chase Cancer Center, Temple Health
Ysabel Duron, Journalist, Cancer Survivor, Patient Advocate, President/Executive Director, The Latino Cancer Institute
Carla Williams, Associate Professor of Medicine & Public Health, Interim Director, Howard University Cancer Center
Bill Hanlon, PhD, Senior Vice President, Head of Real-World Data, Product Strategy, Labcorp
Susan T. Vadaparampil, PhD, MPH, Associate Center Director of Community Outreach, Engagement, & Equity Co-Program Director for Moffitt’s Behavioral Oncology Post Doctoral Training Program, Moffitt Cancer Center
Priscilla Pemu, MD MS, FACP, Professor and Vice Chair, Research, Department of Medicine; Associate
Dean Clinical Research, Director, Clinical Research Center, Morehouse, School of Medicine
Maimah Karmo, Founder & CEO, Tigerlily Foundation
Elizabeth George, Head, Patient Diversity, Labcorp
