Immuno-Oncology Clinical Trials Increased At 17% Average Yearly Growth Between 2008 And 2017, Says GlobalData
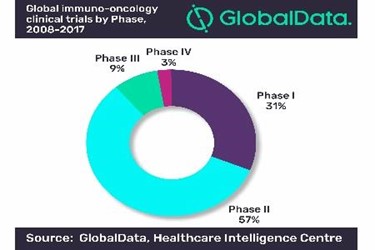
Analysis of global clinical trials in immuno-oncology (IO) for 2008–2017 reveals that they increased at a Compound Annual Growth Rate (CAGR) of 17% over the 10-year period, according to GlobalData, a leading data and analytics company.
For the purposes of this analysis, a small number of Phase 0, Phase I/II, Phase II/III, and Phase III/IV trials were combined with Phase I, Phase II, Phase III, and Phase IV trials, respectively. Overall, Phase II trials outnumbered all other trials by contributing between 52% and 62% across the period, or 57% on average. The share of Phase I trials remained at around 31% across the 10-year period.
Marco Borria, PhD, Healthcare Analyst at GlobalData, comments: “Phase III trials’ contribution ranged between 7% and 12%, or 9% on average, whereas Phase IV trials’ share remained flat at approximately 3%.
“This shows the fast pace IO features with regard to the development of new drugs. It also highlights the relatively early stage the field is in, considering that 88% of the trials are in Phase II or below.”
About GlobalData
4,000 of the world’s largest companies, including over 70% of FTSE 100 and 60% of Fortune 100 companies, make more timely and better business decisions thanks to GlobalData’s unique data, expert analysis and innovative solutions, all in one platform. GlobalData’s mission is to help our clients decode the future to be more successful and innovative across a range of industries, including the healthcare, consumer, retail, financial, technology and professional services sectors.
Source: GlobalData
