Increasing Diversity In Clinical Trials: The Critical Role Of Community Partnerships & Collaborations
By Luther T. Clark, M.D., Madeline Geday, Adrelia Allen, Senaka Peter, Mary Elmer, Kelly Donlon Hoy, and Vivian Ogbonnaya, Merck & Co., Inc.

Clinical trial results provide the critical evidence base for evaluating the safety and efficacy of new medicines and medical products. When the research study population reflects the characteristics of the population for whom treatment is intended, those individuals who subsequently use the medicines are better served and we advance better health outcomes. If clinical trial participants do not reflect the intended use population, results may be less informative and may not be generalizable.
Racial and ethnic minorities in the U.S. continue to be underrepresented in clinical trials — a challenge for both industry and academia. For example, while African Americans and Hispanics/Latinx comprise 13 percent and 18 percent of the U.S. population, respectively, in pivotal trials submitted to the FDA supporting new drug applications from 2007-2017, Blacks (5.4 percent) and Hispanics/Latinx (7.2 percent) were underrepresented, whereas Asians (8.6 percent) were overrepresented compared to their proportion (5.9 percent) of the U.S. population.1-3
The confluence of two major catastrophes that our nation is currently enmeshed in — the COVID-19 pandemic and demonstrations for social justice across the U.S. (triggered by the brutal killing of a Black man at the hands of police officers) — have put a spotlight on systemic racism, healthcare disparities, and the need to promote health equity. The meaningful inclusion of underrepresented communities in the development and conduct of clinical research, as well as equitable access and uptake of new medicines in clinical practice, is imperative if we are to create true progress toward the elimination of healthcare disparities, social justice, and the achievement of health equity.
Barriers To Trial Participation
The major barriers to participation of racially and ethnically underrepresented patients in clinical trials4,5 include: (1) mistrust, (2) fear, (3) lack of comfort with the clinical trial process, (4) lack of information about clinical trials (importance, opportunities to participate, access), (5) logistical constraints associated with participation such as time, out-of-pocket expenses, and other resource constraints, (6) the impacts of social determinants of health, and (7) behavioral change readiness.
In addition, there is a shortage of suitably qualified investigators in underrepresented communities and clinics and a lack of infrastructure and resources needed to perform clinical trials in underrepresented communities and populations. Efforts to improve both of these are foundational to changing the landscape.
Social and economic factors, the social determinants of health (SDOH) (i.e., education, economic stability, neighborhood, health and healthcare access, social and community context), may negatively impact the decision of patients to participate in clinical research.5 Recognizing and understanding the SDOH may help address and overcome the barriers to minority participation in research and ultimately improve the development of healthcare solutions and patient outcomes.5
Overcoming Barriers And Achieving Diversity In Clinical Research
Patient and Community Engagement
Patient and community engagement can be thought of in two separate but related contexts: medical care and research. In the context of medical care, patient and community engagement refers to individuals and communities as active participants in the management of their health, with their preferences and values considered when making medical decisions. In the research context, patient and community engagement is defined by the involvement of patients, caregivers, and patient advocates across all aspects of the research, including formulating research questions, study design, identifying endpoints important to patients, recruitment and retention, as well as the dissemination of the study results.
The goal of patient and community engagement in research is the formation of collaborative partnerships that will ultimately advance both science and patient care.
In addition to helping empower patients to be more active participants in their medical care, these community partnerships and collaborations play critical roles in the development and implementation of effective, sustainable solutions for overcoming barriers to participant diversity — particularly mistrust.6,7 The Yale Center of Clinical Investigation (YCCI) Cultural Ambassadors Program is one example of a highly regarded and effective community partnership7 that deserves emulation and upscaling. The YCCI Cultural Ambassadors Program is a partnership with Junta for Progressive Action and the African Methodist Episcopal Zion (AME Zion) Church that has resulted in broadened community participation in clinical research and increased participant diversity by linking clinical investigators directly to trusted community leaders. This demonstrates that appropriate and meaningful engagement between trusted community stakeholders (participants, patients, caregivers, patient advocacy organizations and leaders) and researchers (clinical trial sponsors, investigators, clinical research sites) can effectively address those issues (perspectives and priorities) that are important for patients, participants, patient groups, and communities — those who will be impacted by the research.
Our clinical studies (Merck & Co., Inc., Kenilworth, NJ, USA) are conducted globally in many different countries and in people of varying age, race, ethnicity, and gender, accounting for other potential disease related factors. Study protocols are assessed for the need and capability to enroll underrepresented groups. We seek to engage underrepresented groups and those disproportionately impacted by the disease under study. We support our clinical trial investigators to enroll underrepresented groups and expand access to those who will ultimately use our products. Where appropriate, and in accordance with U.S. regulatory agency guidance, we make concerted efforts to raise awareness of clinical trial opportunities in various communities. For example, in a pivotal Phase 3 “C-EDGE” trial evaluating the combination of elbasvir/grazoprevir (Zepatier) for treatment of chronic hepatitis C, we demonstrated that even in the presence of significant program challenges (short enrollment period, competitive landscape, aggressive timelines, etc.), enrollment of diverse participants could be achieved and targets exceeded through the use of customized tools and applications such as a dynamic participant enrollment tracker.
In order to gain insights about what matters to patients and their communities, we engage with patients and their advocates through activities such as patient advisory panels and expert input consultations. We have found patient and community engagement to be especially helpful for:
- Increasing awareness and education to build trust and help potential participants and communities understand the importance and benefits of clinical trials
- Increasing opportunities for underrepresented groups to participate in our clinical trials by removing barriers (i.e., out-of-pocket costs).
- Increasing partnerships with minority investigators and those who serve communities of color to help improve the diversity of participants in clinical trials and better demonstrate the safety and efficacy of new drugs, vaccines, and other therapeutic and diagnostic products in all populations.
Trust, Mistrust, and Trustworthiness
Mistrust of research and the research community and fear of being a "guinea pig" are major barriers to clinical trial participation for underrepresented groups. While the ultimate decision regarding clinical trial participation rests with the patient, that decision may also be influenced by family, close friends, and other trusted advisors in their own communities. Therefore, building trust between researchers and trusted community stakeholders (faith-based organizations, nonprofit and civic groups, community leaders, patient advocacy groups, community healthcare providers, etc.) in minority communities is also critical for overcoming barriers. It is also important to note that while altruism and other factors are important considerations, patients are usually most concerned with obtaining the best treatment for their disease. Therefore, reassuring patients about the importance of their health and safety, reinforcing that their health will be closely monitored, and including family members in decision-making can increase comfort with the clinical trial process and help build trust.4 In addition to increasing awareness and overcoming participant and community mistrust, industry sponsors may increase the trustworthiness of their organization and industry through effective partnerships, collaborations, and culturally appropriate communications.4,8
Behavioral Change and Decision-Making
Community engagement can also support the necessary patient decisions and behavioral change readiness associated with overcoming barriers such as mistrust, fear, behavioral biases, etc. Behavioral change does not usually happen quickly and decisively but occurs continuously through a process of readiness and stages. For each stage (precontemplation, contemplation, preparation, action, and maintenance, according to one model), intervention strategies and communications should be appropriately tailored to be most effective advancing from one stage to the next. For example, according to the transtheoretical model of behavioral change, there are five phases4,8 (see figure below), and the decision to participate in a clinical trial is a progression requiring appropriately aligned messages prior to moving to the next phase:
- Precontemplation: The potential patient/participant knows little or nothing about clinical trials and/or is not interested in participating; needs basic information about clinical trials.
- Contemplation: The potential patient/participant has some basic knowledge of clinical research, is willing to learn more, and is willing to consider participation.
- Preparation: The patient/participant has made an appointment with investigators and is asking questions about participation; logistics may become important to discuss during this phase.
- Action: The patient/participant has been pre-screened and has read the consent form; logistics important to discuss during this phase.
- Maintenance: The patient/participant has agreed to participate, signed the consent form, and presents for the baseline appointment; concerns are listened to and addressed.
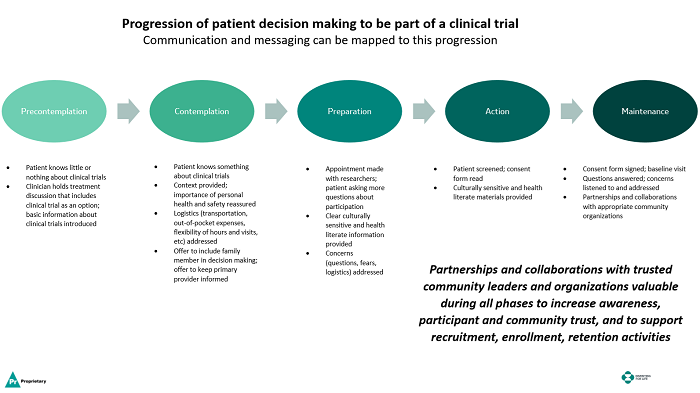
Click the image to view a full-size version.
Conclusion
While the FDA, industry sponsors, academia, and others have taken important steps to address the challenges of clinical trial diversity, progress has been limited. However, during this deeply troubling moment for our nation, the confluence of two major catastrophes — the COVID-19 pandemic and another incident of police brutality sparking protests and community unrest due to racial injustice and inequities — provides perhaps an opportunity to make real progress and accelerate the development of sustainable solutions for achieving diversity and inclusion in clinical research as well as improved healthcare access and outcomes for all communities.
Patient and community engagement, partnerships, and collaborations can play crucial roles in overcoming barriers to clinical trials. Meaningful long-term engagement, collaborations, and partnerships — rather than those based on specific trial or project needs — deepen community relationships, build trust, gain an understanding of community priorities, and make research programs more responsive to the needs, values, and perspectives of the affected populations. Overcoming barriers to recruiting, enrolling, and retaining underrepresented groups in clinical research requires deliberate, concerted, and sustained actions. Community partnerships and collaborations play a critical role in achieving effective and sustainable success.
References:
- New benchmarks quantify demographic under-representation in clinical trials. Tufts Center for the Study of Drug Development Impact Report. Volume 22, Number 3, May/June 2020.
- https://www.census.gov/quickfacts/fact/table/US/IPE120218
- http://www.fda.gov/downloads/Drugs/InformationOnDrugs/UCM570195.pdf
- Clark LT, Watkins L, Pina IL, Elmer M, Akinboboye O, Gorham M, Jamerson B, McCullough C, Pierre C, Polis AB, Puckrein G, Regnante JM. Increasing Diversity in Clinical Trials: Overcoming Critical Barriers. Curr Probl Cardiol 2019; 44:148-172.
- Asare M, Flannery M, Kamen C. Social Determinants of Health: A Framework for Studying Cancer Health Disparities and Minority Participation in Research. Oncol Nurs Forum. 2017 January 02; 44(1): 20–23.
- Weinstein JN, Geller A, Negussie Y, Baciu A. Communities in Action: Pathways to Health Equity. Report of the National Academies of Sciences Engineering Medicine (2017). https://www.ncbi.nlm.nih.gov/books/NBK425848/pdf/Bookshelf_NBK425848.pdf
- Cultural Ambassadors: A Unique Community Partnership.
- Prochaska JO, DiClemente CC. Transtheoretical model of behavioral change. In: Gellman MD, Turner JR, eds. Encyclopedia of Behavioral Medicine. Springer; 2013:97-121.
