Maximizing Immuno-Oncology Clinical Trial Success
By Luke S. Gill, Executive Director, Oncology, Strategic Development, Premier Research
Immuno-oncology is a unique approach to cancer treatment that leverages the body’s immune system to help fight cancer. In recent years, immune checkpoint inhibitors have changed the landscape of immunotherapy, and emerging therapies such as chimeric antigen receptor T-cells (CAR-T), dendritic cell vaccines and bi-specific T-cell engager (BiTE) antibodies are pushing the envelope even further.
In 2016, the cancer immunotherapy market was estimated to be $41 billion, and it is expected to grow to nearly $119 billion by 2025.1 Small and mid-sized biopharmaceutical companies will play a critical role in this growth, but will need to overcome critical hurdles that are inherent in developing immunotherapeutic agents. Because immunotherapy innovations work differently than chemotherapy, they require different standards for evaluating their safety and effectiveness. Understanding these standards—and the other major challenges of immuno-oncology studies—is critical to clinical trial success.
Evaluating Response to Cancer Immunotherapies
Traditionally, response and efficacy with oncology agents has been measured by a set of published rules known as Response Evaluation Criteria in Solid Tumors (RECIST). However, these criteria do not easily apply to immuno-oncology agents because of the kinetics of the anti-tumor response associated them. Unlike conventional cytotoxic therapies that may trigger rapid tumor shrinkage due to direct killing of cancer cells, immuno-oncology drugs stimulate immune cell responses that may take several months to occur. As a result, patients may exhibit an initial increase in tumor burden followed by tumor shrinkage, a phenomenon called the flare effect.
Only applying RECIST criteria to immunotherapy trials can result in:
- Premature termination of therapy
- Unnecessary removal of patients from clinical trials
- Inaccurate interpretations of treatment response
A New Set of Rules: iRECIST
In 2017, a new set of immune-related response criteria was proposed by a RECIST working group comprised of members of industry, academia, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). This consensus guideline—called Immune RECIST (iRECIST)—standardizes and validates immune response criteria to aid in decision-making regarding continuation of therapy in clinical trials.
iRECIST calls for the use of modified RECIST in cancer immunotherapy trials and describes a standardized approach to measuring solid tumors and defining objective change in tumor size for clinical trials.2 iRECIST also introduces a new response criterion known as immune unconfirmed progression of disease (iUPD), which describes new overall response.
With iRECIST, the bar for progression resets if RECIST-defined progressive disease (PD) is followed at the next time point (TP) by tumor shrinkage, as seen in TP2 in the figure below.
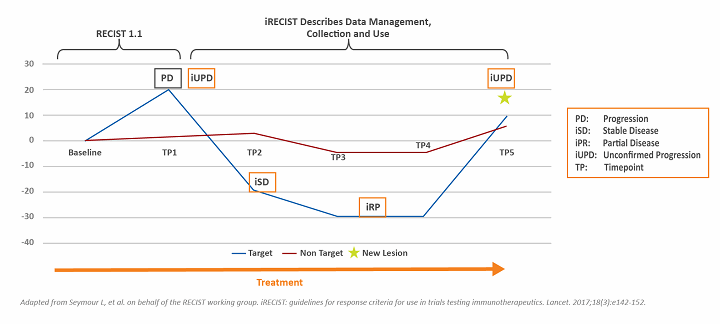
Figure 1: Assessing tumor response using iRECIST
iRECIST has not yet been validated and should not be used as a guideline for treatment decisions. However, iRECIST can be used in conjunction with RECIST in later-phase studies, and may be used as primary response criteria in exploratory, early-phase studies.
Sponsors of cancer immunotherapy drugs who want to use iRECIST guidelines in their studies should train their operational team and communicate closely with the Data and Safety Monitoring Board (DSMB) to ensure that all stakeholders understand that these agents work differently than cytotoxic therapies.
Validating Biomarkers
Current patient response rates and toxicities associated with immunotherapies have created a sense of urgency to determine which patients would most benefit from these agents. To date, the biomarkers for immunotherapy include immunohistochemistry, flow cytometry and next generation sequencing, each of which has its pros and cons. The identification of immune-specific biomarkers will help to fill knowledge gaps by providing valuable predictive and prognostic information, as well as insights on the underlying mechanisms of treatment response and resistance.
A major hurdle to the identification and development of clinically relevant biomarkers is the fact that immune modulation affects many cell types and involves complex interactions among the host, cancer cells and tumor microenvironment.3 The Society for Immunotherapy of Cancer (SITC) Biomarkers Task Force has published a series of white papers on the validation process and regulatory considerations associated with biomarkers in immunotherapy, as well as novel technologies and emerging biomarkers relevant to individualized cancer therapy.
Finding the Right Combination
Cancer treatment is undergoing a radical transformation in which conventional cancer treatments are being integrated with immunotherapeutic agents. Many clinical trials are evaluating the potential synergistic effects associated with immunotherapy drug combinations. However, it has been shown that substantive incremental toxicity can result from these combinations, depending on the patient population, dose and schedule utilized. For example, a phase I study combining ipilimumab with vemurafenib, a Raf inhibitor, in patients with melanoma showed significant increases in toxicity at standard dosing. Combination therapies require not only rigorous clinical testing early in clinical development, but also the willingness to accept the use of non-standard doses or schedules of individual agents to maximize the overall risk-benefit profile.4
Identifying Adverse Events
Especially with the shift toward combination immunotherapy, it is becoming increasingly important for sponsors and investigators to be adept recognizing, characterizing and monitoring immune-related adverse events (irAEs) and other serious adverse events (SAEs).
In general, immunotherapy agents demonstrate unique safety profiles that may differ considerably from most conventional oncology drugs. For example, up to 23 percent of patients treated with ipilimumab develop SAEs, including colitis and hypophysitis.3 When given in conjunction with dacarbazine, approximately 20 percent showed significant elevations of liver function tests.
Sponsors should keep in mind that toxicity does not accurately predict positive therapeutic outcome, and patients may experience irAEs or SAEs without benefiting from an anti-tumor effect. Training trial site staff, as well as patients, caregivers and all members of the healthcare team, how to anticipate, recognize and intervene on irAEs and SAEs will contribute to clinical trial success.
Looking to the Future
Advances in our understanding of the immune response to cancer—along with recent advances in biomarker development—are increasing the number of patients with cancer who benefit from immunotherapy. As we look to the future, new immune-oncology agents and combination approaches have the potential to further expand the spectrum of patients who respond to cancer immunotherapy, improve the quality of clinical responses and pave the way for a personalized approach to cancer treatment.
References:
- Grand View Research. Cancer Immunotherapy Market Analysis by Product, By Cancer Type and Segment Forecasts, 2014-2025. October 2017.
- Seymour L, et al. on behalf of the RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet. 2017;18(3):e142-152.
- Gulley JL, et al. Immunotherapy biomarkers 2016: overcoming the barriers. J Immunother Cancer 2017;5(29).
- Ott PA, et al. Combination therapy: a road map. J Immunother Cancer 2017;5(16).
