'Middle-Out' – Joined-Up Thinking For PKPD Model-Based Learning And Confirming In Drug Development
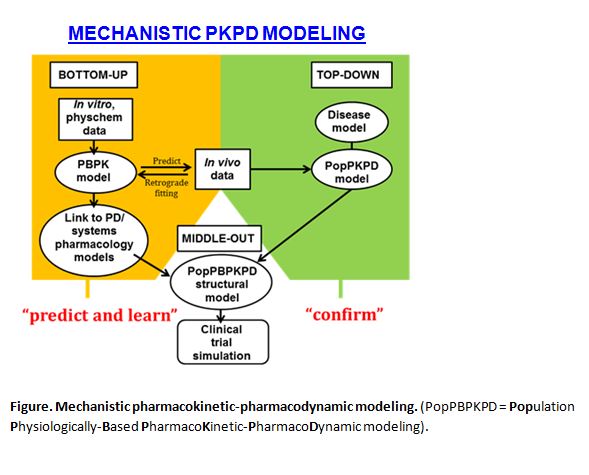
Modeling and simulation have finally come of age in the processes of new drug development and regulation. An integral part of this realization is the increasing application of pharmacokinetic-pharmacodynamic modeling (PKPD), where PK and PD refer to the mathematical description and prediction of what the body does to the drug and what the drug does to the body, respectively. However, there are fundamentally two camps in PKPD modeling, representing ‘bottom-up’ and ‘top-down’ approaches (Figure). The former emphasizes a biological viewpoint while the latter has a strong statistical (pharmacometric) basis.
Different Perspectives
‘Bottom-up’ comes from physiologically-based pharmacokinetic modeling (PBPK) and a systems view of pharmacology . The ‘parts’ are pieced together to predict PKPD behavior in virtual populations defined by demographics, physiology, genetics, disease and co-medication. These parts fall into two categories:
- System parameters including organ sizes, blood flows, the abundance and genetic variants of drug-metabolizing enzymes and transporters, and elements describing the control mechanisms that result in on- and off-target effects
- Drug parameters, including physical chemical properties and in vitro information on absorption, metabolism, transport and receptor-binding
This constitutes a process of understanding; a cost-effective learning domain, informing the intelligent selection and design of subsequent in vivo studies that are invariably hugely expensive.
In contrast, ‘top-down’ starts with the whole body, and seeks to extract information from the in vivo data to identify important covariates affecting drug exposure and response, followed by the design of optimal dosage regimens. This is usually done by population PKPD analysis, often based on sparse sampling in cohorts of patients, and sometimes linked to empirical models of disease progression.
Joining Forces
The challenge is now to align and integrate the ‘bottom-up’ and ‘top-down’ approaches more intimately, combining the rich functionality of the former with the statistical rigor of the latter, while obviating the uncertainty in the former and the limited mechanistic insight afforded by the latter.
Population PKPD should be viewed primarily as a confirming process for the predictions (e.g. of the influence of patient-related covariates) that emerge from physiologically-based PKPD modeling. The middle ground of the two approaches is currently apparent with the construction of minimal, semi-mechanistic structural models in population PKPD and the judicious application of parameter estimation in physiologically-based PKPD.
A true ‘middle-out’ approach (Figure) evaluates the most influential components of a full physiologically-based PKPD model by sensitivity analysis, while fixing the others, as the basis of a more informative and flexible structural model within traditional population PKPD. In this way, the pre-clinical/clinical silos in Pharma can be busted; the power of clinical trial simulations enhanced; and the exciting potential of extending PKPD modeling and simulation to guide point–of–care dosage selection, and personalized medicine can be realized.
ABOUT THE AUTHOR:
Geoff Tucker is the Emeritus Professor of Clinical Pharmacology, University of Sheffield, UK; Vice President of Translational Sciences, Certara; and Chair of the Board of Pharmaceutical Sciences, International Pharmaceutical Federation.
