7 Most Talked About Factors To Transform Clinical Trials
By Ivanna Rosendal, global head of digitalization, Ascendis Pharma and podcast host, “Transformation in Trials”

What does it take to accelerate clinical trials and get drugs to patients sooner? This is a question that I have posed to over 80 experts working within the clinical space as part of my role as the host of Transformation in Trials. In their answers, there are clear trends. These trends, however, do not stand alone in their potential to transform clinical trials; they impact one another with the potential to start a virtuous cycle of transformation. This article will detail the trends and how we embrace them to impact the parts of the complex clinical ecosystem that are within our control.
“One Wish” To Change The Life Sciences Industry
The purpose of Transformation in Trials is to understand the many small ways companies are optimizing clinical — and clinical research professionals are hopefully reusing those learnings in other settings.
The podcast has been running for three years and has welcomed over 80 guests, with 60% from the U.S. and 40% from Europe. Even though each episode focuses on a specific topic, I always ask guests the same question:
“If we gave you the Transformation in Trials magic wand that could change one thing in the life sciences industry, what would you wish to change?”
To my surprise, it is rarely the science or clinical practices that the experts wish to change. It is things closer to our grasp. Things we can all contribute to.
7 Factors For Transformation
Here are the guests’ seven most popular factors, listed in order.
1. Collaboration
When guests call for more collaboration, they usually mean collaboration in multiple ways:
- between pharmaceutical companies
- with regulatory agencies
- across functional siloes
- between sponsors and academia
- with third parties such as digital health providers or patient organizations.
Collaboration ensures the reuse of solutions to tough problems and fosters innovation. For example, Rani Naik, a GCP QA expert, wishes for “[m]ore transparency and sharing of challenges and issues, because we, as an industry, we all face the same challenges.”
If good solutions to common problems could be disseminated across the industry, less time would be lost on reinventing the same solutions.
When it comes to fostering innovation, cross-functional collaboration within a pharmaceutical company could lead to better targeting of a drug, thereby increasing its chance for approval and reimbursement. Collaboration across pharmaceutical companies and other parties could ensure more innovative treatment options altogether.
2. Open-mindedness
The second most called-for change is a move away from conservatism and toward open-mindedness. This call includes attempting process changes as well as the adoption of technology and a data-driven approach.
Kimberly Tableman, founder and CEO of Espero, sums it up, “Embrace change. We need to move quickly and take on innovation, fail forward, take a risk. If we take a protective stance of not trying anything new, the industry can’t move forward.”
Examples of process improvements include collecting multiple endpoints in a Phase 2 trial to determine which pathway to follow in a Phase 3 trial. When it comes to technology changes, guests often wish to abolish the decades-old monolithic systems in favor of tools fit for a specific trial and that enable faster turnaround times. Many guests also wish for improved access to data so scientists can gather insights early and pivot, if necessary.
3. Patient focus
Designing trials with patients in mind — including involving patients in the trial design — is summed up as “patient focus.” André Chagwedera, founder and CEO of Flemming Protocol, wishes for “[p]atients [to have] a tangible and powerful role in the process of deciding and driving the future of healthcare and life sciences.”
Focusing on the needs of patients means considering not just the clinical aspects of the trial but also understanding how the trial fits into their everyday lives to ensure smooth participation and adherence. It also covers the development of treatment options that allow patients to have a constructive, long-term experience with the drug once it is marketed.
4. Incentive model
Drugs are developed and funded depending on both patent structure and drug pricing models. Many guests argue that both the patent structure and the current reimbursement models create perverse incentives that decrease innovation. In our current model, more funding goes to large companies and not necessarily the most innovative or needed drugs. Savva Kerdemelidis and Nicholas Fiorenza both advocate for a change in incentive models so that drug development companies can fund research that most benefits patients.
The key argument is that with alternative incentive models, we can have increased innovation as well as more affordable drugs.
5. Novel treatment
Speaking of innovation, guests often wish for a broader playing field within research. Andy Yates, chief scientific officer at Artelo Biosciences, specifically talking about controlled substances, wished “[t]hat medicine was being brought forward because of the value of the preclinical science and clinical science was treated as a medicine under development instead of a potential abuse liability.”
But it is not just about the compounds used for clinical trials. Guests also desire the ability to do more explorative research on compounds without having to commit to a specific disease area until more evidence is gathered. This can be supported by changes in methods of collecting evidence, more data sharing about compound groups, and databases with drug candidates from academia.
6. Equity in trials
Explorative research might also entail finding narrower groups of patients in which drugs work. This leads us to the topic of avoiding bias in clinical trials. It starts with whom we recruit to participate but also with whom we intend to recruit in the first place. Both Helle Hartnack and Ulla Sofie Lønberg, representing Clinical Operations and Medical Affairs at AbbVie, contest that we need to give patients a fair chance to decide for themselves and not just assume their preferences.
Avoiding bias means including more women, children, and diverse ethnic and socioeconomic groups in clinical trials. This undoubtedly leads us to face challenges that are difficult to solve to ensure the inclusion of these groups. Including groups that would not naturally gravitate towards participating in a clinical trial means more efforts on the sponsors side to include these groups, and tailoring information to them. This can be as low practical as ensuring that trial information is available in the non-dominant language in a country, to providing additional financial support for low-income groups. These are some of the challenges that can be solved through increased cross-industry collaboration.
7. Passion
Industry experts acknowledge that there are no easy answers to many of the bottlenecks that slow down our industry. But getting up every morning and attempting to solve these problems will lead to better results in the long term. Realizing that we may not have the expertise to solve all problems ourselves, some choose to inspire others. Jill Donahue, medical and scientific affairs professional as well as speaker and author, said, “I want to inspire people. To find what lights that fire and connects them to their purpose.”
Other guests call for organizations where scientists can pursue drug development without the constraints of bureaucracy and company politics. Others call for alternative organizational constructs that will allow the pursuit of science over profit.
Enabling Transformation
These seven factors are interesting in isolation, but what is more interesting is how they impact and amplify each other.
First Enabling Loop
If we consider what it would take to enable more collaboration in our industry, I will argue for more open-mindedness. There would be more open-mindedness if the incentive model would allow for more collaboration without fear of punishment or lost profit.
Focusing more on patient needs might incentivize researchers to create more opportunities despite the potential adverse effects of collaboration even in our current climate. Focusing on patients is more likely when open-mindedness is already in place, supported by an incentive model built to solve patient needs, and passionate people willing to collaborate even at the risk of financial detriment. These enabling loops are illustrated below.
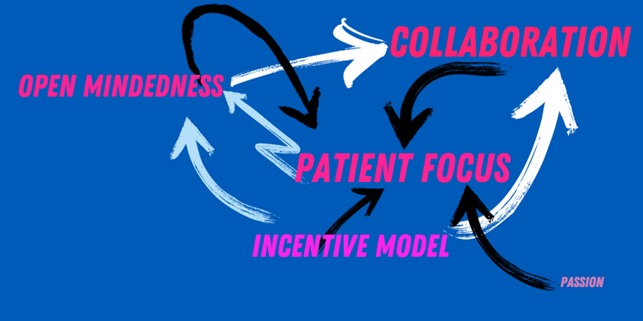
However, the incentive model does lie somewhat outside of this loop. Even though it impacts the other factors, it is not impacted by any of them. Since the incentive model in most countries is bound by the political reality of the country in question, it is not within our reach to change. However, we are also citizens and can advocate for change in incentivizing drug development.
Second Enabling Loop
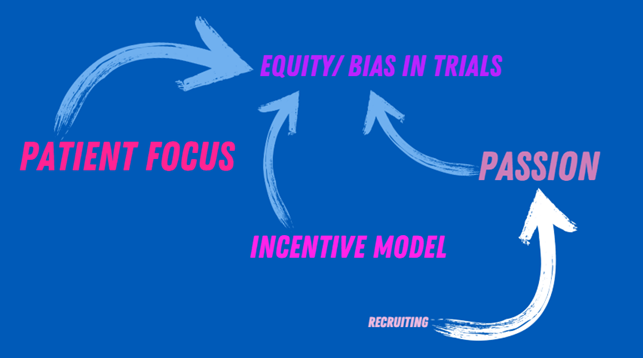
Whereas the first enabling loop focuses on cross-industry collaboration, the second enabling loop centers around creating more equity and avoiding bias in clinical trials. This is enabled by an increased patient focus, supported by an incentive model that emphasizes inclusion. But it is also assisted by people who want to understand the whole patient population. Hiring people with a scientific mindset may help enable this passion.
“One Wish” Is Not Enough
These seven factors are likely to impact the trajectory of our industry. The tricky part is that no one factor can do the trick — and no one wish can transform our industry. Instead, our industry must evolve across all seven factors. The good news is that positively addressing one of the factors will likely impact the others.
This is where we all come into play and where I state my wish: Let us each impact the factor that is within our reach. Perhaps you can advocate for a trial to include a broader range of patients. Perhaps you can collaborate across business functions or even companies. Together, we can nudge trials toward transformation, so patients do not have to wait for treatment.
About The Author:
Ivanna Rosendal is the global head of digitalization at Ascendis Pharma and a behavioral economist. She has worked toward optimizing clinical trials in large and small biopharmaceutical companies for the past 15 years. Now she focuses on leveraging technology to enhance the work of scientists, clinicians, and medical teams, ensuring they can make a significant impact on patient outcomes. Through initiatives like her podcast, Transformations in Trials, she aims to make life sciences more accessible and foster collaboration to drive meaningful change.
