Navigate DCTs Thoughtfully To Improve Patient Experience & Increase Trial Accessibility

By Life Science Connect Editorial Staff
Decentralized clinical trials (DCTs), which leverage digital and remote technologies, are exploding across the pharmaceutical industry for a variety of indications. Given our increasing global reliance on digital tools, it is likely that we are only seeing the beginning of how this trend affords greater accessibility to patient populations. At Sanofi Digital Technologies, a global pharmaceutical company, researchers have implemented digital components across 100% of their trials, providing ample opportunity to assess how DCTs impact the patient experience in terms of burden, efficacy, and accessibility. By leveraging patient-centric trial design and digital technology, they are building a strategy to ensure that DCTs benefit as many patients as possible.
Setting A DCT Strategy With Digital Data Collection
According to a recent webinar survey, drug sponsors are now conducting DCTs across a range of therapeutic areas, with neurology and oncology leading the pack. Currently, Sanofi’s pharmaceutical pipeline includes therapeutics in oncology, neurology, rare diseases, and vaccines. In order to map their DCT strategy, Sanofi assesses what a particular trial should emphasize, the data it should gather, and the best ways to measure it. Some trials are heavily focused on outcomes; in these scenarios, Sanofi closely tracks sleep and daily activity and is beginning to measure pain levels. In other trials, the emphasis is placed on remote data collection or endpoints.
DCTs rely on digital biomarkers (DBMs), which are defined by Sanofi as “objective, quantifiable measures of physiology and/or behavior used as indicators of biological, pathological processes or responses to exposures or interventions that are derived from digital measures.” DBMs are assessed using wearable devices, smartphones, or other digital tools and can replace or supplement traditional biomarkers and clinical outcome assessments. Common DBMs include cognition, physical activity, motor function, mobility, heart rate, sleep, and respiration. Sanofi leverages digital data collection and DBMs during DCTs to serve three distinct purposes, all of which have associated activity tracking (Figure 1):
- to monitor patient safety from home and ensure that clinical trials can be decentralized without losing safety oversight,
- to determine whether a trial can be taken into a patient’s home in terms of endpoint collection, and
- to enhance patients’ overall quality of life.
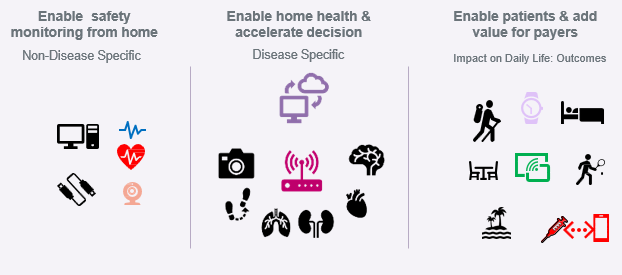
Figure 1. Sanofi’s purposes for digital data collection for DCTs
Navigating The Inevitable Challenges
The initial DCT challenge is identifying an ideal device for the measurements needed. At the outset, Sanofi considers, “What do we want to measure?”, which is more complex than it sounds. For example, if you want to track daily patient activity, you must determine if you want to track steps, active hours, or the distance they cover. To assess the practicality of different devices, stakeholders conduct tests to evaluate the user experience of recording data in the proposed fashion.
To that point, patient burden is a major challenge and concern. Though DCTs seek to make the experience easier on patients, every trial comes with different obstacles. Potential burdens include asking patients to complete paperwork, visit the clinic, wear devices, and upload data. At Sanofi, care teams are considering how to minimize these to ensure that they do not become exclusion factors for diverse patient populations.
One way to minimize the burden is providing support. Though some patients prefer to use their own devices, Sanofi also provides devices to patients who need them. There are various reasons a patient may require or prefer to use a provided device, including not wanting to share medical data on a work device or not having the data capabilities needed to transfer data on their personal device.
It is also crucial to implement mechanisms that account for any unforeseen circumstances that might compromise data integrity. During one Sanofi trial, a patient’s sleep device which was placed under the mattress displayed an irregular heart rate; it turned out that the patient’s sick child was lying in her parents’ bed, causing the device to pick up her heart rate instead of the patient’s. Situations like this are a vital example of how data or device efficiency could be compromised during a DCT. To ensure that there are not complications for patients using digital devices, there must be close data oversight by the investigator. Integrated devices can also help, including a skin patch that conducts a bio scan to confirm data is being retrieved from the trial participant.
Validating A Digital Endpoint
To use a digital device in a Phase 3 trial, researchers should begin thinking about validation while setting the disease area strategy. A digital endpoint has not yet been used as a primary endpoint in a Phase 3 trial; it will likely take years of commitment and research to validate and secure regulatory approval. Even if the goal is to use a digital assessment as a secondary endpoint, it needs to start early. One strategy to achieve this is non-interventional studies that test and validate a device. Another mechanism is using Phase 1 and Phase 2 trials to validate the device that might become an endpoint in Phase 3. Sanofi does this by running validation studies with clinic and home assessments; the results must demonstrate that the at-home data has the same validity as the clinic data.
Leveraging The Power of Digital Technology
People have rapidly adapted to conducting everyday activities through apps, including accessing their healthcare providers and medical records. DCTs are leveraging these technologies to afford patients greater flexibility, convenience, and accessibility as they participate in potentially lifesaving therapeutic programs. At Sanofi, clinicians and researchers are striving to provide patients with a single point of entry for their DCT needs: an integrated platform where they can collect data, review relevant study instructions, change appointments, and schedule drug delivery. By affording patients this level of access, sponsors have the chance to make a far greater impact on global health and well-being.
The above content originally appeared on the Clinical Leader Live podcast; watch the full episode here.
