Number Of Immuno-Oncology (IO) Trials In China Surge By More Than 50% In 2016 And 2017, Finds GlobalData
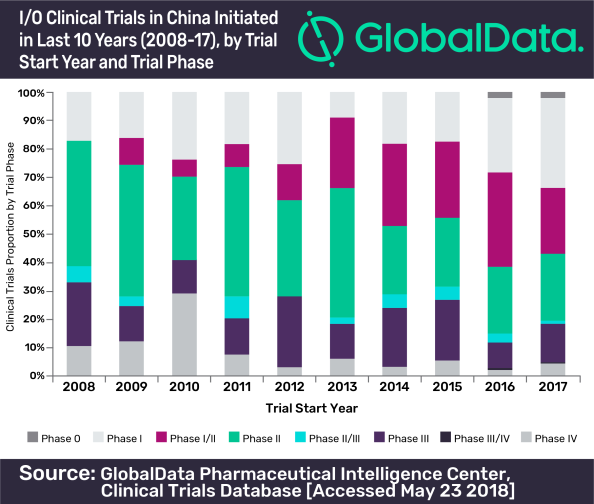
In recent years, China increased its focus on the clinical trial investigation of immuno-oncology (I/O) drug candidates. The number of clinical trials recorded a compound annual growth rate (CAGR) of ~34% since 2008, according to leading data and analytics company GlobalData.
GlobalData identified 819 clinical trials that have been initiated in the last 10 years (2008-17) to investigate I/O drug candidates. More than half (n=464) of these trials were initiated in 2016 and 2017.
Phase II clinical trials accounted for nearly 28% of the total clinical trials conducted in China, followed by Phase I (24%) and Phase I/II (24%).
In the I/O clinical trials, 67% of the trials were in progress, 24% were completed and 5% were planned. Out of these completed trials, 51% were reported with results.
FUDA Cancer Hospital is the major non-industry player investigating I/O drug candidates in China. The other top non-industry sponsors were China PLA General Hospital, Sun Yat-sen University.
Jiangsu Chia-tai Tianqing Pharmaceutical Co Ltd emerged as the top industry sponsor. Other top industry sponsors were Bristol-Myers Squibb Co., F. Hoffmann-La Roche Ltd and Novartis AG.
The major indications under investigation include Non-Small Cell Lung Cancer, Solid Tumor, Hepatocellular Carcinoma, Acute Lymphocytic Leukemia (ALL, Acute Lymphoblastic Leukemia), and Breast Cancer, etc.
The top five I/O drug candidates investigated in the clinical trials that were started in recent years (2016-17) include Cellular Immunotherapy for Oncology, tucidinostat, SHR-1210, nivolumab and anlotinib hydrochloride.
- Information based on GlobalData’s Clinical Trials Database
- This report was built using data and information sourced from proprietary databases, primary and secondary research, and in-house analysis conducted by GlobalData’s team of industryexperts.
About GlobalData’s Clinical Trials Database
The Clinical Trials Database is a comprehensive source of accurate and detailed intelligence on global Clinical Trials, covering a wide range of Therapy Areas and Indications, including Rare Diseases. Intelligence is compiled by experts on Clinical Trials conducted all over the globe and in various phases of development, with data crossed-checked with key registries, conferences, associations, journals, and regulatory websites
About GlobalData
4,000 of the world’s largest companies, including over 70% of FTSE 100 and 60% of Fortune 100 companies, make more timely and better business decisions thanks to GlobalData’s unique data, expert analysis and innovative solutions, all in one platform. GlobalData’s mission is to help our clients decode the future to be more successful and innovative across a range of industries, including the healthcare, consumer, retail, financial, technology and professional services sectors.
Source: GlobalData
