Nurturing Clinical Site Relationships Drives Trial Success
By Life Science Connect Editorial Staff

The biggest obstacles to strong relationships between sponsors, CROs, and clinical trial sites include poor communication, contract/budget negotiation delays, and difficulty procuring timely or accurate data. In recent years, site networks have introduced strategies and infrastructure to help alleviate some of these concerns, but the power to further strengthen these partnerships is in the hands of leadership.
Collaboration Lifts All Sites
For years, the number of clinical trials has been outpacing the professionals available to conduct them. Consequently, seamless collaboration and transparent communication among sponsors, CROs, and research sites are now essential. This interconnectedness empowers individual sites and networks to manage this ever-growing number of clinical trials. A collaborative environment also improves each site’s trial participation experience, strengthening the site itself and supporting the PI ecosystem.
Moreover, sites are stronger collectively, so sharing best practices with one another is an obvious advantage, fostering widespread improvement and efficiency across the clinical trial landscape. Sites occasionally reach out to one another for advice about processes, regulations, or technologies — a practice that is encouraged by many industry stakeholders. In fact, the Society for Clinical Research Sites (SCRS) provides an open platform that allows sites to ask questions, share SOPs and exchange intelligence — a vital resource that makes each site more competitive from a selection standpoint. Being honest and collaborative with each other can also provide sites with leads on upcoming trials.
Importantly, though, communication dynamics suffer when messages or data are improperly contextualized: if everything is urgent, nothing is urgent. Most sponsors/CROs attempt to introduce context by soliciting partner feedback in various ways, from surveys to interpersonal interactions at sites, and then act on the feedback accordingly. CRA turnover and other difficult-to-predict events also can lead to suboptimal communication dynamics, but the point is to strive for a constant cycle of improvement. Sponsors/CROs should regularly evaluate site challenges as well as pore over communications for accuracy, timeliness, and appropriate urgency.
Start Small And Build Toward Constant Improvement
Partnerships start small. For a sponsor, this generally means seeking new and/or unfamiliar sites in every trial. As qualifying metrics, predictability in performance and communication are positive signs of potential for a deeper, long-lasting partnership. Predictability must be a two-way street between sponsors and their site/network partners.
While the PI historically has been viewed as the key source of trust at any given site, sponsors are increasingly open to working with trial-naïve investigators if site infrastructure and support staff can be confirmed adequate. Site capability and potential may be determined by looking at site executives’ expertise, support staff experience, site infrastructure, and site quality oversight.
Indeed, ICH GCP E6(R3) suggests that sponsors gather input from the people who will be implementing a trial — including sites, research support partners, and patients — as they design the protocol. Additionally, some sites perform mock visits to help the sponsor discover potential logistical issues. Wherever both patients and sites can be involved, protocol designs are much more representative of all parties’ concerns and priorities.
Sponsors also benefit when sites can detail attrition relevant to support staff, as well as contingency plans if staff or infrastructure are suddenly compromised or lost. And, while sponsor companies typically do not investigate a site’s financial health as part of feasibility and qualification, that concern has grown in recent years. If a site goes out of business, it leaves behind numerous patients for the sponsor to sort out.
Site teams that can clearly describe their expectations of the sponsor help establish a strong foundation for the future of the partnership. This courtesy allows both parties to determine whether the sponsor is a reliable collaborator for the site (not just vice versa). For example, some sites may prefer to limit regular in-person meetings and the associated disruptions with a sponsor that repeatedly sends different people to review the same data. One way sites can address this issue is to incorporate a fee into their contract to account for the time spent accommodating redundant sponsor visits.
Additionally, it's crucial that site teams proactively establish their value and communicate their needs and perspectives clearly from the outset of any collaboration. Oftentimes, they can be reluctant to reach out to the sponsor or CRO, especially since smaller sites often partner with business development organizations that act as brokers or act as part of a network. But once the relationship begins, site teams are responsible for continuing to nurture those relationships.
One effective strategy is for sponsor/CRO and site leadership to meet regularly to review expectations and the metrics used to gauge them. These routine discussions enable partners to understand the factors influencing outcome delivery, including any failures, as standard metrics may not always show the full picture. As a loose guide, the Sand Cone model depicts how to improve organizational performance, starting with quality as a foundational layer and then moving to building predictability.
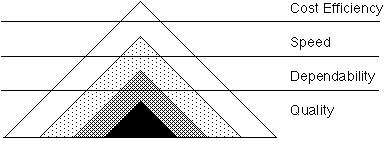
Fig. 1 — The Sand Cone model suggests that, although it is possible to trade off the four key capabilities in the short term, a hierarchy exists among them.1
Related, particularly for sites dealing with large sponsors or CROs, it is critical to understand the sponsor’s escalation pathway early in the relationship. Some sites don’t realize the appropriate timing or triggers for escalation or know the extent to which escalation is possible (i.e., how far “up the ladder” they need to go). Other sites may fear a punitive response: If they escalate, will they be overlooked for subsequent trials? From a sponsor’s perspective, it is crucial to ensure that a research site’s experience aligns with the company’s intended image as a collaborative and reliable partner.
Even Within A Network, Each Site Should Receive Individual Attention
Site networks streamline partnerships by offering sponsors and CROs a unified corporate structure, access to a single contract/budget negotiator, and a lead organizational monitor working across all sites. Site networks also enable the use of identical — or very similar — processes and technologies at each site, potentially driving faster and more efficient operations than would be possible with a disparate collection of sites.
Regardless of whether a sponsor/CRO works with individual sites, a network, or some combination of the two, an increasing number of organizations have created specific departments to nurture both group and individual site partnerships. For example, Merck maintains “tiers” of relationships with numerous sites and networks. The top tier includes a small number of elite sites with whom Merck books a substantial amount of business because those sites consistently provide high-quality performance.
Moreover, Merck is among the sponsors that ensure site needs are not overlooked by assigning site relationship managers who are not transactional trial-to-trial but instead work through institution-to-institution issues with the site, frequently reporting back to the sponsor’s leadership. Metrics collected by both sides can be used to inform a partnership structure and to confirm all parties are meeting their commitment. Merck erected a similar structure around contract and budget negotiations. The company’s leadership has stated that Merck maintains numerous site partnerships by prioritizing person-to-person relationships and open communications.
Similarly, Sanofi has an initiative aimed at facilitating site investigations by creating a working group to assist individual sites in addressing challenges as they arise. For example, how can a sponsor or CRO actively involve a research site during the protocol development stage? Or, what can a sponsor or CRO offer to enhance a site’s enrollment initiatives, such as funding community engagement initiatives or implementing an AI tool that can be integrated with the EMR?
Driving Site Performance
Unfortunately, “wearing multiple hats” at a site is a common occurrence. Of course, patient care remains the top priority, but sites that are hiring also must prioritize stakeholders, as they are also important “customers.” For example, the research coordinator needs to be organized and detail-oriented, and the site should understand how that potential employee’s goals align with trial outcomes.
It also is helpful for sites to have insight into sponsor planning, because it directly affects how the site will use its budget toward staffing and other endeavors. Clinical research demands that sponsors, CROs and sites possess the foresight to accurately anticipate what might happen a year or more in the future.
This information is not always easy for sponsors to predict, but failure to try has led to many delays and even study cancellations. Sites tend to experience more sustainable and scalable growth using this strategy, rather than trying to assign more responsibilities to an already over-burdened team. As a result, communication with sponsors improves and the site is likely to win more trials as its performance metrics trend upward.
In the case of site networks, a point person who represents multiple sites eliminates waste and redundancy by reducing the administrative burden on others. Troubleshooting is assigned to task experts or specific roles instituted to manage those situations, freeing other personnel to focus on enrollment and compassionate patient care. In terms of technology’s impact on sponsor/CRO partnerships, less is more. Specifically, so much technology is available that it often creates a bottleneck for sites.
Additionally, it is vital that site networks are able to describe exactly how each technology they utilize improves their operational efficiency, quality, and/or cost. Evaluations of technology must be conducted on a site-by-site basis. While some sponsors may offer their preferred technologies, allowing sites to use programs, systems, and workflows with which they are already familiar minimizes potential complications and risk.
Not all “upgrades” offer practical benefits, and the rapid pace of innovation means new technologies are continually emerging. The goal is to keep the technology’s concept consistent across the trial while still enabling flexibility in how each site uses that technology.
Agree On Outcomes, Collaborate On Solutions
Maintaining strong relationships between sponsors, their CROs, and clinical trial sites requires constant effort, occasional investment, and ingenuity. A key product of these inputs is high predictability within the relationship and deep understanding of each partner’s needs and motivations. This awareness, in turn, leads to efficiency and translates into cost savings. To learn more, watch the Clinical Leader Live event examining ways to create and maintain robust sponsor/CRO-site partnerships.
1University of Cambridge Institute for Manufacturing “Trade-Off Models.” University of Cambridge. https://www.ifm.eng.cam.ac.uk/research/dstools/trade-off-models/
