Pairing Conversion Rates With Patient Personas To Better Understand Enrollment Challenges
By Tirrell Payton, managing partner, Nooma Group Consulting

Once upon a time, there was a study manager named Julie. Julie worked at Aeonis Life Sciences. She loved the company and loved her job. Julie was assigned to manage a critical study for C3 glomerulopathy.
For months, Julie and her team worked tirelessly to accelerate enrollment. Despite a series of targeted marketing campaigns, patient outreach programs, and collaborations with clinical research organizations, nothing seemed to work. Pressure began to mount, and frustration simmered within the team as they watched their enrollment numbers lag behind the projected timeline.
They focused efforts on driving awareness, following the logic that more awareness would naturally translate to higher enrollment numbers, and invested heavily in search engine optimization, pay-per-click advertising, and partnerships with patient advocacy groups to spread the word about their C3 glomerulopathy study. Unbeknownst to Julie and her team, the root cause of their problem lay deeper in the patient journey.
A closer look at the major touchpoints in the patient journey revealed that the real issue was not awareness but rather a significant dropout rate after prescreening but before consent. Patients who had initially expressed interest and even gone through the prescreening were, for reasons unknown to Julie and her team, not consenting.
As they continued to struggle with enrollment, the atmosphere grew tense. Team meetings were frustrating: Everyone knew the implications of missing the enrollment targets. A blame storm was forming. Upper management grew increasingly impatient, and the threat of losing funding loomed large.
If Julie's team had leveraged a more comprehensive approach, incorporating both quantitative data (such as enrollment numbers and dropout rates) and qualitative insights (such as understanding patients' motivations, concerns, and barriers to participation at each key touchpoint), they might have identified the true issue and tailored their strategies accordingly. By addressing the actual root cause, they could have mitigated the risk of missing enrollment targets, fostering a more collaborative and productive team atmosphere and ultimately preserving funding for the critical study.
Supplementing Quantitative Learnings With Qualitive Insights
Let's start by defining some of the tools Julie's team could have used to drive both quantitative and qualitative insights. Analytical tools are software and methodologies used to analyze and interpret data, helping identify patterns, trends, and insights. This is generally what people think about when they think about quantitative insights. On the other hand, empathy tools such as patient personas, empathy maps, and patient journey maps help drive qualitative insights that may be otherwise overlooked. They can be used individually or in combination to improve enrollment and retention rates and are powerful when used in conjunction with quantitative techniques.
Now, let’s take a look at the stages of a simplified patient enrollment journey:
- Awareness: The stage at which a patient learns about the existence of a clinical trial as a potential treatment option for their condition.
- Identification: The process of determining whether a patient meets the preliminary eligibility criteria to be considered for participation in a clinical trial.
- Prescreening: A stage where a potential trial participant undergoes further evaluation, including medical tests, to confirm their eligibility for the clinical trial.
- Consent: The point at which a patient, after understanding the risks and benefits, formally agrees to participate in the clinical trial by signing the informed consent form.
- Eligibility testing: The point at which a series of tests and evaluations are conducted to verify that a patient meets all the required inclusion and exclusion criteria before officially enrolling in a clinical trial.
- Enrollment: The final stage where a patient is officially registered as a participant in the clinical trial and begins receiving treatment or intervention as part of the study.
When patients move from one stage to another, it’s called conversion. (And when they don’t convert, that’s dropout.) Expectedly, fewer people make it to the end of the journey than begin it. When that data is visualized, it appears as a funnel, or an inverted pyramid, with more people coming in at the top and fewer remaining at the bottom. To understand how many people complete each stage of the process, it’s important to calculate an average conversion rate. Then, to better understand the behavior of cohorts, you baseline the specific cohort (e.g., Hispanic females) and see how/where their conversion rates deviate the most from the average.
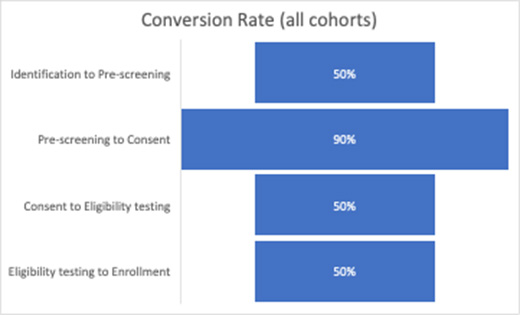
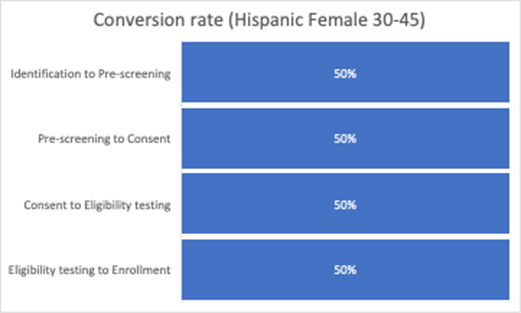
In this example based on Julie’s experience, we see that the Hispanic females aged 30 to 45 cohort has a significantly lower prescreening to consent conversion rate compared to the average. While we have used the quantitative data to tell us where the conversion rate is lower for this cohort, we must use qualitative tools to help us understand why. This is where patient personas come in.
Using Patient Personas To Better Understand Conversion Data
A patient persona is a semi-fictional representation of a target patient population based on demographic information, behaviors, needs, and motivations. By creating detailed patient personas, you can better understand the unique perspectives and concerns of different patient groups, enabling you to tailor your trial design, communication, and recruitment strategies to their specific needs.
To better understand Julie’s study experience, let’s create a persona of Yolanda, a 35-year-old C3 glomerulopathy patient. Yolanda is:
- heavily reliant on a support community for opinions, perspectives, and decision-making help,
- fluent in English but prefers documentation in Spanish so that she can socialize with her support community, and
- a bit of a trailblazer in that she has stepped outside her usual community healthcare resources to participate in a clinical trial.
When following Yolanda through the journey of what happens after prescreening and before consent, we lay out a few milestones along the journey.
As Yolanda transitions from the prescreening stage of the clinical trial enrollment process, she is filled with a mixture of hope, anxiety, and curiosity. Having successfully passed preliminary evaluations, she finds herself on the brink of a significant decision that could potentially alter the course of her treatment journey.
Yolanda is not alone in navigating this journey. She heavily relies on her support community – family, friends, and fellow kidney patients – to help her process her experiences and make informed decisions. During medical appointments, she often has a close friend or family member accompany her, and she frequently discusses her experiences with her wider support network, seeking their perspectives and insights.
As a trailblazer, Yolanda values being informed and understanding all aspects of her treatment options. When she receives the informed consent form, she intends to share it with her support community in Spanish, which is her preferred language for discussing such critical matters. She wants to ensure she fully comprehends the nuances of the document and can discuss it openly with her community.
However, the consent form is presented only in English. This presents a significant emotional pain point for Yolanda. Her trust in the clinical trial process is shaken by the lack of consideration for her language preferences, making her feel marginalized and unsupported. Furthermore, this language barrier limits her ability to share and discuss the document with her community, denying her the opportunity to seek their invaluable advice and support.
As a result, despite her initial enthusiasm for participating in the trial, Yolanda ultimately decides not to consent. The lack of a Spanish-language consent form was a significant barrier, making her feel excluded from fully participating in the decision-making process. It highlights a need for better accommodation of diverse patient needs in clinical trial processes, particularly in regard to language and cultural considerations.
From this experience, we see an obvious and glaring issue: The consent form is not available in Spanish. The cohort analysis helped us understand where dropouts were occurring (prescreening to consent) and among which population (Hispanic females aged 30 to 45), as well as provide the insights into the journey that helped us understand why (consent form not available in Spanish).
Armed with insights from our patient journey, not only can we ensure consent forms are available in Spanish but also that we are thoughtful about the patient journey as it relates to all the needs identified by our patient persona and our patient journey.
How To Craft Patient Personas
Using qualitative tools to understand patient experience is a fantastic way to augment analytical insights. Here are some factors to consider both when crafting personas as well as understanding which qualitative insights to collect:
When crafting your personas:
- Details matter: For each persona, outline their demographic information, goals, pain points, motivators, and most importantly, how they make decisions.
- Take your persona on a journey: Understand the journey that patients go through in making enrollment and consent decisions. This can provide key insights into touchpoints and opportunities for recruitment.
- Ask real patients: Include actual patients in the persona development process. Their first-hand experience can provide invaluable insights that you might not get from purely hypothetical scenarios.
When identifying insights to collect:
- Identify common themes: Look for patterns or recurring themes in the patient experience. These might include beliefs, attitudes, experiences, or behaviors that multiple participants share.
- Prioritize actionable insights: Some insights will be more actionable than others. Prioritize those where you have the ability to make changes. For instance, if patients express a need for more flexible visit schedules, that's something you might be able to address.
- Use quantitative data for validation: Where possible, use quantitative data to validate and prioritize qualitative insights. For example, if a certain perception about clinical trials is common among a large proportion of your target population, it might be a priority to address.
Patient personas are not meant to stereotype or oversimplify, and qualitative insights are not definitive proof, but both work together to guide you in understanding patient needs and potential barriers to recruitment. Use them as part of a broader, patient-centered recruitment strategy that also includes quantitative data and ongoing evaluation.
 About The Author:
About The Author:
Tirrell Payton is the managing partner at Nooma Group Consulting, where he helps life science leaders innovate. Prior to founding Nooma, Tirrell served as a leader in McKinsey’s life science practice. He holds an MBA from Northwestern’s Kellogg School of Management and lives in sunny San Diego with his wife and 6 children.
