3 Patient-Centric Steps to Increase Retention

By Bruno Gagnon, B.Pharm, MSc, Executive Consultant, Comprehend
Patient centricity is a growing trend in our industry. From the creation of Chief Patient Officer positions to culture-change initiatives, one of the strategies to improve the quality of clinical trials is to treat patients as “consumers” rather than “subjects” in a study. Most of these efforts hope to bridge the gap between the biopharma industry and people living with a disease.
To date, Clinical Operations (ClinOps) has only been a small part of these efforts. But there is huge potential for ClinOps to increase the patient centricity of study execution, and, in turn, the satisfaction of patients.
There are three primary benefits of taking a patient-centric approach to Clinical Operations. First, ClinOps can reduce leakage through the enrollment process to increase retention and reduce the pressure on recruiting new patients. Second, ClinOps is uniquely positioned to manage patient protocol compliance to avoid costly amendments and ensure that patients remain in the trial as long as they stand to benefit from it. Finally, with access to real-time data, ClinOps can quickly respond to any safety and logistical issues by dedicating additional resources to studies and sites most in need.
Three benefits of a patient-centric approach to Clinical Operations

For the purpose of this post, we are focusing on retention, and its implications for completing trials on time, within budget, and with high patient satisfaction.
The Other Side of Enrollment: The Retention Challenge
In clinical development, most enrollment efforts focus on recruitment. Less often discussed, however, is the other side of the enrollment challenge: patient retention.
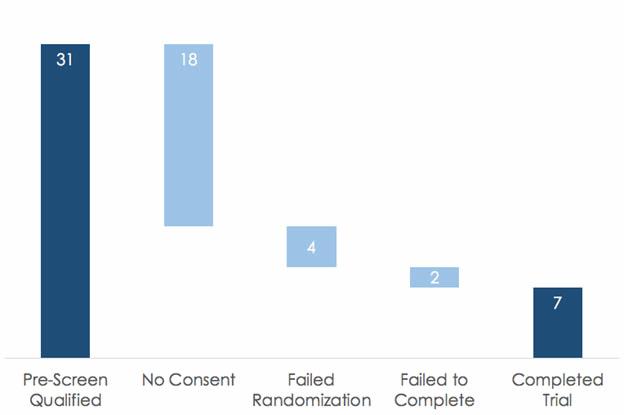
Recruiting customers is tough - only 31 out of every 100 eligible and available patients will make it to the pre-screen qualified stage in the enrollment funnel of a clinical trial. But of these 31 patients, only 7 will complete the trial. It is therefore no surprise that 85% of clinical trials fail to retain enough patients. There are serious consequences of dropouts – from costly delays to missing data that can compromise study results.
Out of every 31 qualified patients, 7 complete the average trial
At the same time, sponsors are struggling to improve the patient experience. A recent survey by Memorial Sloan Kettering suggests that only 40% of Americans have a positive impression of clinical trials and only 35% are “likely” to enroll in a clinical trial.
Is there a path to overcoming the patient retention challenge, while improving the patient experience?
3 Steps to Increase Retention
When considering patient retention best practices, let’s draw on the experience of one Vice President of ClinOps. At the time, he oversaw seventeen studies with a team of five and one Contract Research Organization (CRO) at a mid-sized biotechnology company.
Critically, this VP had invested in Clinical Intelligence, a class of software to manage disparate data sources and keep his decentralized team on the same page. Clinical Intelligence aggregated data from multiple sources, delivered cross-study insights, enabled one-click site-specific investigation, and facilitated cross-team collaboration to ensure he met his milestones and keep his trials on-time and under budget.
To meet new corporate patient-centricity standards, he and his team were paying particular attention to patient retention in their studies. To address retention - and improve the patient experience - this VP created a simple three-step playbook.
Step 1: Diagnose the leakage in the enrollment funnel
First, he recognized that he needed full visibility to each step of the enrollment process. Without understanding where patients were falling out of the enrollment funnel, it would be impossible to know how to improve retention.
Clinical Intelligence typically includes out-of-the-box key performance indicators, including an enrollment funnel that he was able to use to track progress of patients through each stage in the process. A glance at his dashboard showed that this particular study was experiencing a higher-than-expected withdrawal rate.
Step 2: Quickly investigate the root cause
Why were so many patients withdrawing from the study? With a single click, the VP was able to directly investigate the root cause of withdrawals. Visualization of withdrawals by type over time showed him that most patients were designated, “Lost to Follow Up.” Unlike “Safety” or “Death” designations, “Lost to Follow Up” is typically a process issue that can be addressed, which left him optimistic that his team could resolve it.
One additional click revealed the countries most responsible for a high proportion of these designations, and realized that Lebanon in particular was experiencing an issue.
Step 3: Close the loop from insight to action
A time series of withdrawals in Lebanon showed that there was one early death and another early safety issue at two of the sites. After these incidents, he saw an uptick in patients withdrawing and being designated “Lost to Follow Up.” Intuitively, it seemed that patients were failing to follow up due to concern with these early adverse events. But he needed to confirm this with his team.
Within Clinical Intelligence, he assigned a task to his team to confirm the issue and create a plan for proactively educating patients regarding the early adverse events.
His intuition was right, and proactive education of patients empowered them to make a more informed decision. As a result, more patients decided to stay in the trial, and the proportion of patients who withdrew from the study was reduced.
This proactive, data-driven approach to trial management is proven to reduce the likelihood of missed milestones. Less often discussed, however, is how this approach will also improve the patient experience. By identifying a withdrawal-rate issue in real time, this ClinOps team addressed the issue before it led to a trial delay. And by directing resources to these problem sites, they ensured that patients had the information they needed to make an informed decision about whether to participate in the trial.
On-Time Clinical Trials and Happier Patients Go Hand-in-Hand
ClinOps has an important role to play in improving the patient experience. With access to accurate and timely insights on key patient-related indicators - powered by Clinical Intelligence - Biopharma professionals can act quicker to resolve issues before they more broadly impact the trial. This proactive approach to issue management will simultaneously improve the patient experience and overcome the patient retention challenge.
