Patient Trust In Pharma Is Low; Here's How To Rebuild It
By Shalome Sine and Annick de Bruin, CISCRP

This article is part of a quarterly series highlighting key insights from a global longitudinal survey study of over 12,800 patients and members of the public conducted in 2025. In this article, we will review what the survey results show about patient and public levels of trust in pharmaceutical companies, as well as specific strategies pharmaceutical companies can take to rebuild trust.
The low level of trust in pharmaceutical companies among patients and the public has long been acknowledged as a key issue that is widespread and has persisted for so long that it may seem like an unsolvable challenge, too large in scale to have concrete solutions that would realistically be impactful.
Our study, however, suggests that while public trust in pharmaceutical companies remains low, patients and the public are receptive to actionable, achievable, and tangible trust-building strategies that sponsors, operational teams, and others in the industry can implement to rebuild trust step by step.
Brief Methodology Overview
Since 2013, the Center for Information and Study on Clinical Research Participation (CISCRP) has conducted the Perceptions and Insights (P&I) Study, a global longitudinal survey study assessing public and patient attitudes toward, and experiences participating in, clinical research. In 2025, CISCRP’s P&I Study surveyed 12,887 respondents around the world. This article highlights select findings related to trust and trust-building between patients and pharmaceutical companies.
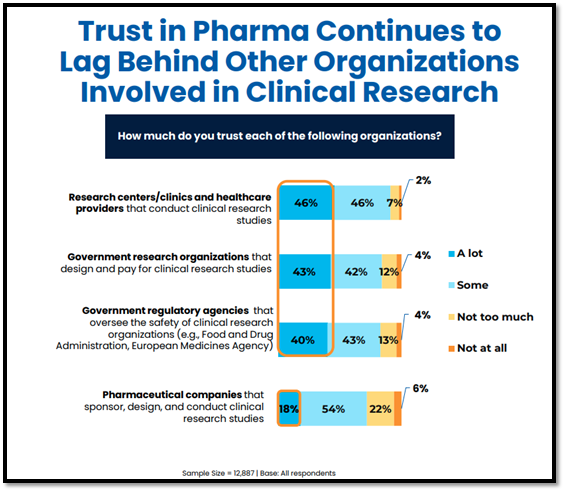
Trust In Pharma Lags Behind Other Clinical Research Organizations
Compared to government research organizations, government regulatory agencies, and independent sites/clinicians, trust in pharmaceutical companies lags significantly. Results from the 2025 P&I Study show that only 18% of respondents globally trust pharmaceutical companies “a lot,” compared to 40% or more who indicate that they trust government agencies/research organizations and research centers/healthcare providers “a lot.”
These findings are generally consistent with findings from past years of the P&I Study, highlighting a measurable and persistent lack of trust in pharmaceutical companies and a significant opportunity to rebuild trust levels.
This lower level of trust may stem from several factors, including a perception that pharmaceutical companies are primarily profit-oriented, which can raise concerns about potential conflicts of interest. In the 2021 P&I Study, among respondents who indicated low trust in pharmaceutical companies, the top cited reason for distrust was “because pharmaceutical companies only want to make money” (65%, among n=2,946 individuals who indicated “not at all” or “not much” trust in pharmaceutical companies).
Strategies For Building Trust
However, findings also indicate that while the issue of low trust in pharma is persistent, it may not be intractable. CISCRP’s 2025 P&I study also investigated public and patient receptivity to various trust-building strategies.
Offer Key Clinical Trial Information In Plain Language
As in past years, respondents continue to indicate that transparency about the drug development process is key to trust-building. Sixty percent felt that their level of trust could increase if the company shared more information about the health risks and benefits of their medicines, and 50% believed their level of trust in pharma would increase if the company shared more information about prior completed clinical research on their medicines.
Pharma companies can also build trust among patients and the public by sharing information about the company’s steps to improve diverse representation among clinical trial participants as well as study staff. Among those identifying as Black/African American, American Indian or Alaska Native, and Asian/Pacific Islander, “knowing that the company included a diverse set of participants in their clinical studies and employed staff that was diverse” was a top-mentioned trust-building strategy.
Engage Patients In The Clinical Trial Design Process
Involving patients in the clinical trial design and development process was also cited as a top trust-building strategy. Forty-nine percent of all respondents believed that their level of trust would increase by knowing that the company actively works with patients, caregivers, and patient communities to make clinical research studies easier to participate in.

Provide Accessible Education And Engagement To Patients And Their Families
Higher levels of familiarity with the clinical trial process are associated with greater levels of trust in pharmaceutical companies. The 2025 P&I findings show that high trust in pharma is positively associated with self-reported understanding of the term “clinical research/clinical trial.” Trust levels are also higher among clinical trial participants (23% trusting “a lot”) compared to those who have never participated in a clinical trial before (15% trusting “a lot”). These findings indicate that educational interventions to raise awareness and understanding of clinical trials may raise trust levels.
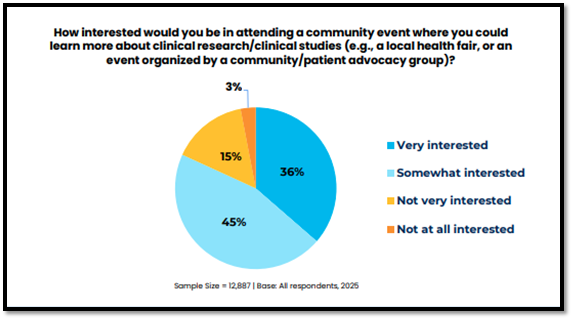
As such, the 2025 P&I study explored receptivity to attending an educational initiative, such as a community event where patients and members of the public could learn more about clinical research. Interest levels in attending this kind of event were high, with the majority (81%) indicating that they would be “somewhat” or “very interested” in attending.
Interest levels in attending an educational community event were highest among those who were Hispanic/Latino and those residing in Africa, South America, and North America, and they were especially high among those who were Black/African American (60% would be “very interested”) and those who were American Indian or Alaska Native (43% would be “very interested”).
Enable Healthcare Providers To Meaningfully Discuss Trial Participation With Their Patients
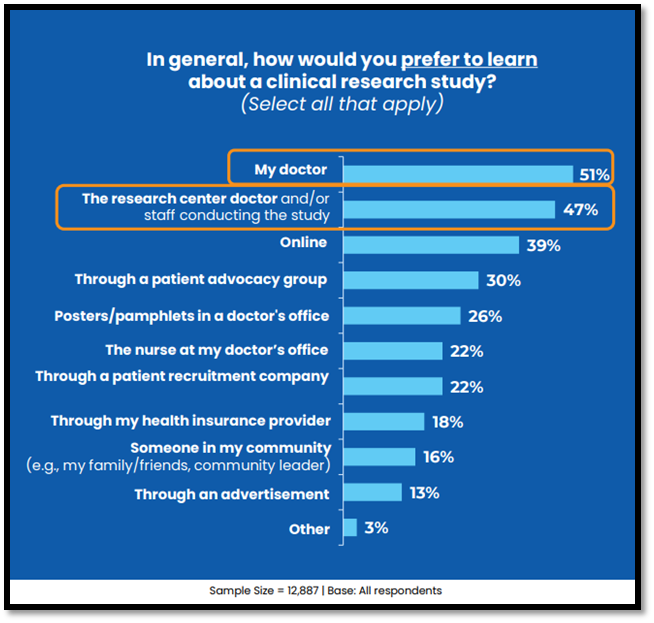
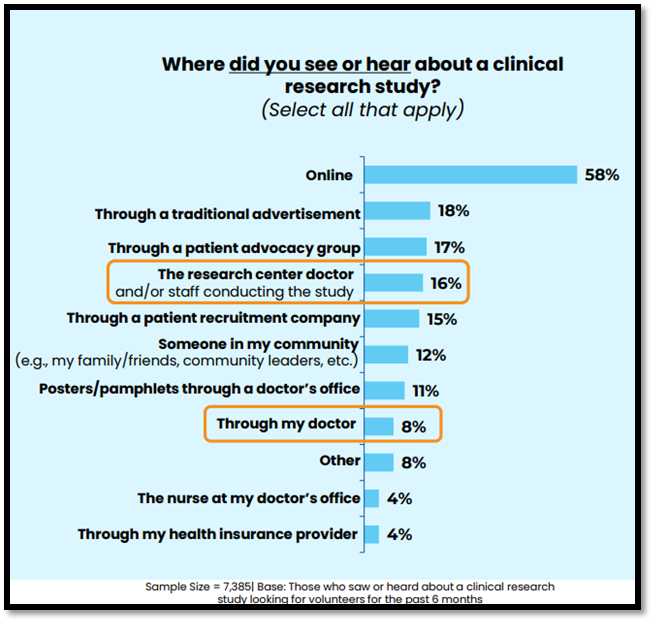
Doctors and other healthcare providers (HCPs) represent trusted sources of health information, and many patients rely on their care teams to inform them of all available treatment options, including clinical trial participation opportunities. However, 2025 P&I results show that, though the majority of respondents would prefer to learn about a clinical trial from their doctor (51%) or the study doctor (47%), most of those who have heard about clinical trials recruiting new participants learned about these opportunities online (58%). These findings are generally consistent with past years, representing an ongoing disconnect between how patients would prefer to learn about clinical trials (e.g., through doctors/HCPs) versus how they actually learn about clinical trials (most often online or through advertisements).
This is an opportunity for pharmaceutical companies to directly engage with HCPs and prepare them with both general and specific information and materials needed to support doctor-patient conversations about clinical trials. For example, trial sponsors can provide HCPs with timely and concise information about new and recruiting clinical trials, including more technical HCP-facing details as well as plain language materials for patients. These materials help HCPs inform their patients about clinical trials and recommend specific participation opportunities where appropriate.
Looking Ahead: Pathway To Elevated Trust In Pharma
While trust levels in pharmaceutical companies remain low, the 2025 P&I results continue to show high levels of interest in clinical trial participation opportunities. For example, the majority (87%) reported that they would be “somewhat” or “very willing” to participate in a clinical trial, and this high level of willingness is consistent across all subgroups by race and ethnicity, with at least 73% in every group indicating that they would be “somewhat” or “very willing” to participate. This reveals an opportunity for pharmaceutical companies to better connect with a broad range of patients who are already interested in clinical trials and generally willing to participate, if given the opportunity.
By maintaining transparency regarding treatment risks, benefits, and past study results, engaging with patients and the public through patient advisory activities and community events, and encouraging conversations about clinical trials between patients and their doctors, pharmaceutical companies can meaningfully build trust. Trust-building initiatives like these can further enable pharmaceutical companies to respond to the high level of interest in learning more about clinical research and strong willingness to participate in clinical trials that already exist among patients and the public.
For more information on the Perceptions and Insights Study and to review the full 2025 findings reports, please click here.
Stay tuned for the next installment of our quarterly series highlighting more findings from this global study.
About The Authors:
 Annick de Bruin is the chief research and insights officer at the Center for Information and Study on Clinical Research Participation (CISCRP). She is responsible for the design, implementation, analysis, and reporting of a variety of CISCRP research studies, including the Perceptions & Insights studies and numerous patient advisory boards. She has more than 25 years of experience conducting primary and secondary research studies in the healthcare, life sciences, and consumer goods industries. She holds an MBA from the Graduate School of Management at Boston University and a Bachelor of Science degree from Bryant University.
Annick de Bruin is the chief research and insights officer at the Center for Information and Study on Clinical Research Participation (CISCRP). She is responsible for the design, implementation, analysis, and reporting of a variety of CISCRP research studies, including the Perceptions & Insights studies and numerous patient advisory boards. She has more than 25 years of experience conducting primary and secondary research studies in the healthcare, life sciences, and consumer goods industries. She holds an MBA from the Graduate School of Management at Boston University and a Bachelor of Science degree from Bryant University.
 Shalome Sine is a senior manager and quantitative insights specialist at the Center for Information and Study on Clinical Research Participation (CISCRP), where she leads efforts to elevate the patient voice in clinical research. Shalome believes that patient perspectives are essential to designing inclusive and efficient clinical trials. As part of CISCRP’s research services team, Shalome oversees both quantitative and qualitative patient voice initiatives, with a specialization in survey-based research that captures insights from patients and the public. She holds a Master of Public Health from Tufts University.
Shalome Sine is a senior manager and quantitative insights specialist at the Center for Information and Study on Clinical Research Participation (CISCRP), where she leads efforts to elevate the patient voice in clinical research. Shalome believes that patient perspectives are essential to designing inclusive and efficient clinical trials. As part of CISCRP’s research services team, Shalome oversees both quantitative and qualitative patient voice initiatives, with a specialization in survey-based research that captures insights from patients and the public. She holds a Master of Public Health from Tufts University.
