Pfizer's Palbociclib Is The Top Breast Cancer Drug Used In Clinical Trials Initiated In 2018, Says Globaldata
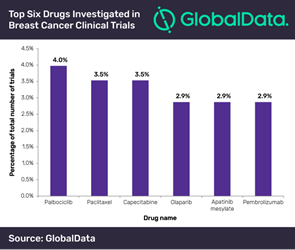
Breast cancer is the most commonly diagnosed cancer in women and is the fifth largest cause of cancer mortality worldwide. Accordingly, a substantial amount of research is geared towards investigating effective therapies. Against this backdrop, Pfizer’s palbociclib–a cyclin-dependent kinase (CDK) inhibitor with high anti-neoplastic activity, emerged as the top drug used in 4% of breast cancer studies initiated in 2018, according to GlobalData, a leading data and analytics company.
GlobalData analysed the number of clinical trials, examining breast cancer with start dates between 1 January 2018 and 16 November 2018 and found that the clinical trials involving breast cancer accounted for 10.9% of all oncology trials initiated in the specified time frame.
The majority of clinical trials were in Phase II (50.8%), with non-industry sponsors taking the lead and accounting for 57.8% of trials. China, with 10.6% of the total clinical trials, was second only to the US (22.4%) during the review period. Biomarkers were used in 22.7% of trials, with cytokine-related biomarkers being the most commonly researched (17%).
Mohamed Abukar, Pharma Analyst at GlobalData, says: “The vast majority of trials investigating palbociclib use combination therapy (83%) to assess progression-free survival. The tubulin inhibitor paclitaxel was investigated in 3.5% of clinical trials, primarily in combination regimens; only 6.3% of clinical trials investigate the agent as monotherapy, and involved comparing different routes of administration.”
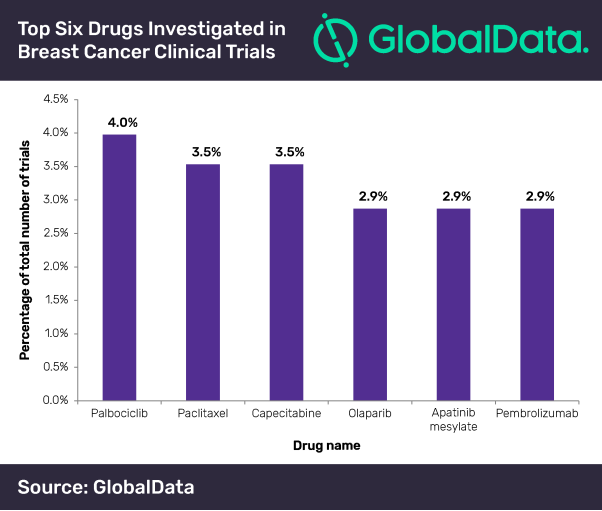
An equivalent number of trials (3.5%) investigated capecitabine. Of note, F. Hoffmann-La Roche’s Phase III study focuses on early relapsing recurrent triple-negative breast cancer. Olaparib, apatinib mesylate and pembrolizumab also made the top six with an equivalent number of clinical trials (2.9%).
Abukar concludes, “Trials involving the poly (ADP-ribose) polymerase inhibitor olaparib focus predominantly on metastatic breast cancer (76.9%). Furthermore, all trials using the tyrosine kinase inhibitor apatinib mesylate were in Phases II or IV with locations only in China, while the majority of trials examining the antineoplastic immune-modulating agent pembrolizumab are being carried out in the US and do not span beyond Phase II.”
About GlobalData
4,000 of the world’s largest companies, including over 70% of FTSE 100 and 60% of Fortune 100 companies, make more timely and better business decisions thanks to GlobalData’s unique data, expert analysis and innovative solutions, all in one platform. GlobalData’s mission is to help our clients decode the future to be more successful and innovative across a range of industries, including the healthcare, consumer, retail, financial, technology and professional services sectors.
Source: GlobalData
