RBM Implementation: Developing An Effective Risk Based Monitoring Plan

By Ashok Ghone, Ph.D., Vice President, global services, MakroCare USA
Risk assessment and risk management are two key aspects of Risk-Based Monitoring (RBM) implementation. RBM is about taking a holistic approach to assess all possible risks related to your study/program and then having a proper plan for risk management which includes systematic monitoring and controlling/mitigating risks throughout the conduct of a study/ program. The correct identification and assessment of study-specific risks, categorization, and implementation of risk-based study-specific monitoring plans are critical components to ensure high quality, integrity of data, enhanced human subject protection, building operational efficiencies, and cost optimization. The monitoring plan should provide the clear direction on how one would monitor and control/mitigate high, medium, and low risks related to data, study conduct, and clinical investigations using various monitoring approaches (centralized, on-site, and off-site). The complexity of the study design, the target disease population, the end points assessments, the phase of the study, geographical area of the study, and the technology used are important factors to consider when developing an effective monitoring plan.
Key Aspects Of An RBM Plan:
- Defining Key Risk Indicators: An RBM plan should define the key risk indicators, their metrics, and the thresholds for corrective actions based on the performed risk identification and assessment.
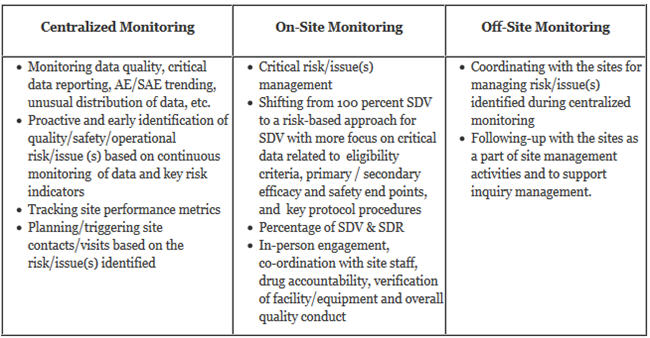
- Describing Monitoring Approaches: A risk-based monitoring plan is different than the traditional monitoring plan, as it uses various monitoring approaches like centralized monitoring, on-site monitoring, and off-site monitoring, and also shares the responsibilities with various cross-functional resources. It should clearly describe the monitoring approaches to be used in regard to what should be achieved through centralized monitoring and what should be done during on-site visits and off-site monitoring for controlling and mitigating various types of risk/issue(s). An RBM plan is considered a “dynamic” plan, as the frequency and extent of on-site and off-site monitoring activities will vary based on the critical risk/ issue(s) findings, performance of the sites, the quality of data coming in from different sites, and the identification of new risks. For instance, the sites that are not performing well or are having more quality issues will receive more attention or on-site visits than the sites which are performing as, or better than, expected. An RBM plan must also suggest the process to manage unresolved key issues or significant instances of sites’ non-compliance.
- Roles & Responsibilities Of Various Functional Areas: Development and implementation of an RBM plan requires expertise from cross-functional areas like clinical operations, the medical team, the data management team, biostatisticians, and the technology group. The roles and responsibilities of a central monitoring team, on-site monitors, data management, and technology groups should be clearly defined for successful implementation of risk-based monitoring.
- Communication Plan & Documentation: The RBM plan must describe the system/tools used for documenting centralized monitoring activities. It should clearly outline the communication plan for various stakeholders and the documentation process for centralized monitoring reports, issue escalation, coordination between the centralized monitoring team with on-site monitors or relevant stake holders for risk control or issues resolution. An appropriate communication plan is crucial for global studies. Similarly, efficient coordination among the data management, technology, and centralized monitoring teams is important to ensure the timely identification of risks. The RBM plan must also mention the events where a plan needs to be revised based on the identification of new risks and amendments to protocol, etc.
- Technology Used: The RBM plan should include information about the technology/tools used and the various sources that provided the relevant data to create planned analytics reports to monitor risk/issue(s) related to data quality, patient safety, and trial operations.
- Overall Quality Management: The plan should explain training requirements for the altered process, especially with the introduction of a centralized monitoring process. The monitoring of quality, efficiencies metrics, and planning audits are helpful in building overall quality and in evaluating the RBM implementation process.
Using proper and validated technology and working with sites to ensure timely data entry and a comprehensive data management plan that is in line with the monitoring plan are essential aspects to developing and implementing an RBM approach.
Ashok Ghone, Ph.D. is Vice President, global services at MakroCare USA. He has 20 years of experience in the pharmaceutical and clinical research industry. Ashok has a strong understanding of global clinical research with hands-on experience in clinical operations, project management, clinical trial management, process development, site management, and patient recruitment activities. He has led various cross-functional teams successfully by providing strategic direction and guidance to accomplish local, regional, and global projects involving early- and late- phase clinical studies in various therapeutic areas. Ashok has been involved in the development of processes, systems, and training programs related to risk-based monitoring and centralized monitoring at MakroCare, which offers these specialized services to biopharmaceutical and medical device companies to support their endeavors in the implementation of the RBM approach.
Email: ashok.ghone@makrocare.com
