Real-World Studies: Unique Considerations For Patient Engagement And Retention
By Krista A. Payne, MEd , Vice President Late Phase Studies, Real-World Evidence, Evidera
Patient-centric methods and approaches are integral to the design and execution of both interventional and non-interventional studies.1,2 From within the tightly controlled clinical trial environment to the real-world setting, data that provide patient insights on treatment outcomes and unmet clinical and humanistic need constitute critical evidence necessary for the successful market launch of novel and effective medicines.2 However, without the successful engagement and retention of patients over the full duration of a study period, the quality and completeness of patient-generated insights and study data are at risk. Not surprising, the focus on “patients first” has become a critical component of early planning for study success.3,4
If we define engagement as those design features or activities that elicit the patient’s interest in a study and that inspire their willingness to enroll and actively participate, then making the study relevant and meaningful to participants, including patients directly in the design process, and minimizing data collection burden, are study success factors of paramount importance. With respect to patient retention, once a patient chooses to participate, study processes and related activities must be patient-centric and serve to spark and sustain the patient’s interest and motivation to complete the study as required. Retention strategies are numerous and diverse and can include the development of patient communities or discussion forums, access to disease and health and wellness resources, to fair market compensation for time spent attending study visits, and in the case of clinical trials, important access to novel treatments. Particularly in clinical trials, study visit reminders to reduce confusion and participation burden are also commonplace. The actual engagement and retention strategies and solutions employed will vary based on such factors as study type, design parameters such as duration and assessment schedule, as well as patient characteristics and disease manifestations.
For methodological reasons, patient-centric study engagement and retention solutions appropriate for clinical trials may not always be suitable for real-world studies.
In clinical trials, study protocols mandate study visits at fixed time points and pre-defined intervals to evaluate and compare drug efficacy across treatments. Frequently, a full suite of patient retention and support services spanning telephone or electronic visit reminders, to concierge-style transportation services and comfort kits that minimize burden and achieve complete data for all patients at all trial time points is employed. These approaches aim to ensure that a target sample size of patients attend all protocol-defined visits, and that all data are collected, to permit high quality and sufficiently powered analyses.
Patient retention strategies and solutions in interventional studies do not impact the integrity of the trial design, nor the study results, as the trials are designed to achieve high internal validity under already artificial and highly controlled experimental conditions.
On the other hand, in real-world studies and registries, where drug effectiveness is the focus of investigation, it is paramount, methodologically, to avoid protocolmandated study visits and patient retention strategies that could potentially alter real-world physician and patient behaviors. If, for example, the aim of the study is to better understand patterns of usual care and drug effectiveness and tolerability, then non-persistence to treatment, and missed medical appointments are, by nature, key outcomes of interest. In this scenario, the provision of multiple reminders and transportation to the study site to enhance patient engagement and data quality may actually result in improved treatment adherence – not as a function of the treatment itself, but rather as a result of aspects of the study protocol or related procedures. While minimizing patient burden is a hallmark of a patient-centric study, care must be taken in real-world studies to minimize the extent to which the engagement and retention of patients alters naturalistic behaviors and negatively impacts the external validity, or generalizability, of the results.
If solutions are NOT tailored to the observational study paradigm, then the integrity of the study data and results can be significantly compromised and applications for the use of these real-world data will be limited.
Differences between clinical trials and observational studies that have implications for the development and application of patient identification, retention, and engagement strategies are summarized in Table 1.
Table 1. Summary of Key Differences Between Interventional and Non-Interventional Studies that have Implications for Patient Recruitment, Retention and Engagement Strategies and Solutions.
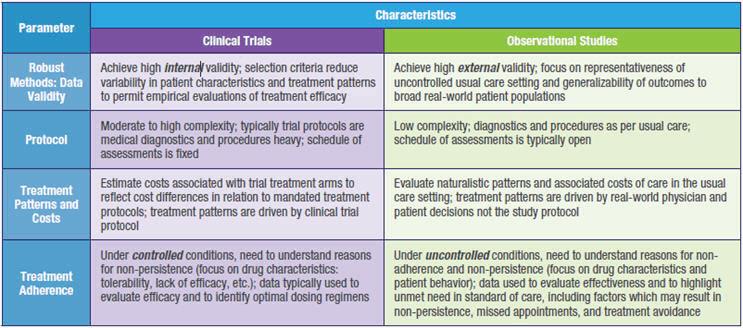
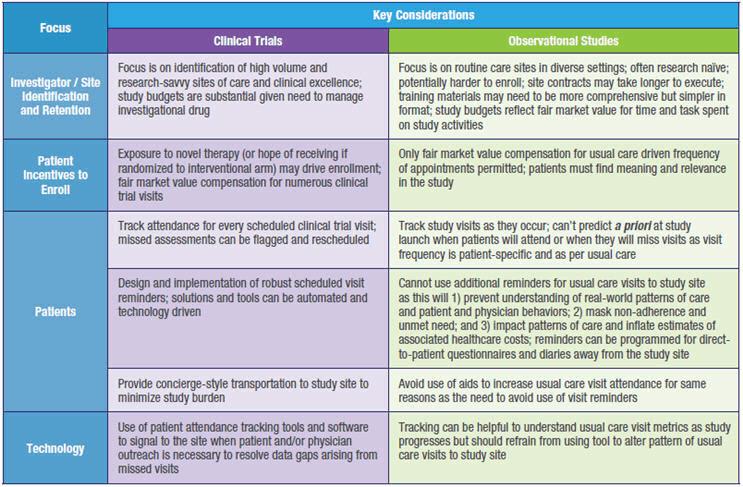
As a result of these fundamental methodological differences, non-interventional prospective studies and registries require engagement and retention solutions that can be markedly different than those applicable to interventional clinical trials. Key considerations for the development of real-world strategies and solutions are presented in Table 2.
Despite some inherent challenges, there are numerous important and effective over-arching strategies for engagement and retention of patients in real-world studies that can be implemented without necessarily impacting the integrity and external validity of the observational data collected.
- • Consider the involvement of patients and/or caregivers in the study design process to better understand what may inspire patients to enroll, anticipate “pain points” for participants, and to inform the development or selection of study outcomes5
- Partner proactively with patient advocacy groups and other resources to
- Inform study design and objectives
- Align study with real-world, community-based resources that can provide information and support to patients and their families
- Establish study e-forums or on-line communities for study patients to connect with each other and share experiences
- Consider employment of patient-centric on-line data entry platforms or “hubs” that integrate data collection with patient access to health and wellness links and other “connectivity” functions
- Minimize study participation burden for investigators and patients through simple and streamlined study protocols and related study procedures <>
Leverage technology to collect data directly from patients separate and apart from usual care visits with consideration given to “Bring-Your-Own-Device” (BYOD) approaches
In summary, a commitment to robust methods to achieve high quality and representative data does not mean that patient-centric study engagement and retention strategies cannot be employed for real-world studies. Careful consideration, however, of the trade-offs between the natural desire to control for complete study data at regular time intervals and adherence to core principles of realworld research that aim to avoid interference with usual care is clearly warranted.
REFERENCES
- Hoos A, Anderson J, et al. Partnering with Patients in the Development and Lifecycle of Medicines: A Call for Action. Ther Innov Regul Sci. 2015 Nov;49(6):929-939.
- The 21ST Century Cures Act. 2016. Public Law 114-255, 114TH Congress, December 13, 2016. Available at: https://www.congress.gov/114/plaws/publ255/PLAW-114publ255.pdf. Accessed October 2, 2017.
- Domecq JP, Prutsky G, Elraiya T, et al. Patient Engagement in Research: A Systematic Review. BMC Health Services Research. 2014;14:89. Available at: https://bmchealthservres.biomedcentral.com/articles/10.1186/1472-6963-14-89. Accessed October 6, 2017.
- Smith SK, Selig W, Harker M, Roberts JN, Hesterlee S, Leventhal D, Klein R, Patrick-Lake B, Abernethy AP. Patient Engagement Practices in Clinical Research among Patient Groups, Industry, and Academia in the United States: A Survey. PLoS One. 2015 Oct 14;10(10): e0140232. doi:10.1371/journal.pone.0140232.
- Geissler JG, Ryll B, Leto de Priolo S, Uhnlenhopp M. Improving Patient Involvement in Medicines Research and Development: A Practical Roadmap. Ther Innov Regul Sci. 2017; 51(5):612-619.
