Sponsors Must Act Now to Mitigate the Impact of COVID-19 Slowdown
Dr. Gen Li, President, Phesi

As we progress through the COVID-19 pandemic, governments, societies and businesses are having to rapidly develop long-term plans to deal with the crisis. Globally, economies are having to make huge, once-in-a-generation adjustments, and there is no doubt that the ramifications will be unpacked for years to come in all industries. While the biopharmaceuticals sector has naturally taken center stage during recent events, it too is feeling the effects of the worldwide slowdown. From early-phase research to manufacturing and post-market surveillance, the impact of shelter-in-place orders and ‘lockdown’ conditions is substantial. Nowhere is this truer than within the clinical trials domain, where there is a heavy reliance on in-person interactions.
However, as we get used to the idea that social distancing will continue throughout 2020 and potentially beyond, rapid action is needed to minimize the impact of the pandemic on clinical trial infrastructure and to adjust to this ‘new normal’. As the immediate crisis transitions into a semi-permanent state, biopharmaceutical companies and trial sponsors must recognize the importance of continued funding of non-COVID-19 related trials. While the virus is dominating agendas now, other, vital research projects must also be protected. Trial sponsors need to take steps now to work around the limitations that COVID-19 imposes and crucially, ensure clinical trials can continue.
Site suspensions on the rise
As of April 2020, according to ClinicalTrials.gov, there were 337,235 clinical studies registered in 211 countries.[1] Yet recent data show cause for concern. There has been a 10 percent increase in site suspensions over the past two months, according to a recent global analysis covering more than 300,000 sites by Phesi (see image). This timeframe coincides with the effects of worldwide shutdowns, which have seen normal life in almost all regions disrupted beyond recognition.
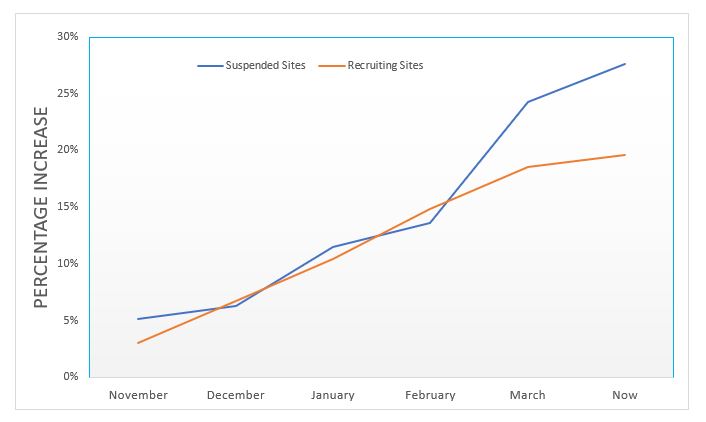
With many staff at biopharmaceutical firms furloughed, labs and research institutions closed, travel restrictions in place, and participants worried about interacting with healthcare professionals – it’s not surprising that trials have taken a hit, but it is certainly alarming. It costs at least $30,000 to activate a site and takes an average of 100 days for an investigator site to be operational; during which time, a number of processes must be followed to ensure appropriate governance and compliance measures are taken, including Institutional Review Board (IRB) approval. It is imperative now that sponsors and CROs recognize their responsibilities in both addressing the risk of closures and suspensions, and helping sites remain financially viable.
The implications of sites being suspended are severe for all stakeholders. For patients and trial participants, the disruption is clear during what is already an uncertain time, particularly for those with underlying health conditions. For independent trial sites, even temporary closures cause financial threat as they continue to incur infrastructure costs without any revenue coming in – this ultimately can lead to their permanent closure. For the sponsor, there is the issue of mounting costs and time as they are forced to open new sites, and there’s also the impact on the quality of the study through loss of patient data or trial results; the knock-on effect of this data discontinuity causes additional package approval challenges to be navigated.
An industry challenge
To achieve this, the answer for sponsors and CROs is simple – they must continue to funnel money to sites, even those where trials have been suspended, to keep them operational. Interim payments will only total relatively small amounts to a large biopharmaceutical company but will make a huge difference to the site itself and ensure it can keep running. In addition, rather than demanding that contracted milestones are met, sponsors must relax their rules around holdback funds given the unprecedented circumstances; this is also something that the Society for Clinical Research Sites (SCRS) has recently called for.[2]
In the world of clinical research, what should be a symbiotic relationship between sponsors and sites, is typically dominated by the sponsor or CRO. We know that the environment pre-COVID-19 was already very difficult for sites and many were in a precarious position. Cash reserves, for instance, were not a luxury – unlike many of their sponsors – that they could afford. Indeed, the SCRS found as recently as 2019 that almost two thirds of sites (59 percent) have only three months or less of operating cash.[3] Large biopharmaceutical firms are at the center of a huge ecosystem, and it’s important they support their partners in these unprecedented times. A small amount of funding now will prevent additional trial costs spiraling post-COVID-19.
Biopharmaceutical companies must consider the bigger picture – the impact of COVID-19 on trials is not just a challenge for the site operator, it is a challenge for the industry. By helping their partners to keep functioning, sponsors are helping themselves, protecting trial outcomes and maintaining their integrity. Safeguarding existing projects by continuing to fund them, and supporting all clinical trial infrastructure, will be essential for when we do emerge from the other side of the pandemic.
[1] Total number of registered clinical studies recorded by ClinicalTrials.gov, US National Library of Medicine, accessed April 27th, 2020
[2] SCRS Calls on Sponsors to Release Holdback Funds in Response to COVID-19, SCRS, April 2nd, 2020
[3] Ibid.
