3 Steps For More Effective Sponsor-CRO Partnerships

By Rick Morrison, CEO, Comprehend Systems
Clinical Operations (ClinOps) leaders increasingly outsource critical components of their trials. By one estimate, 75% of pharmaceutical and biotechnology companies are now outsourcing data management and over 70% are outsourcing site operations. As a consequence of the growth of this practice, effective collaboration between Sponsors and their CROs has never been more critical for achieving clinical trial milestones on-time and within budget.
What is CRO Oversight & Collaboration?
This question is frequently asked by experienced ClinOps managers. CRO Oversight & Collaboration is an ongoing challenge. In the 2016 ClinOps Benchmark Report, these managers told us that their CRO relationships are often colored by costly change orders that are caused by preventable enrollment, site productivity, and subject compliance issues.
What causes these issues? In this same Benchmark Report, teams describe their approach to CRO Oversight & Collaboration as resource-intensive, manual, reactive, and, subsequently, unsuccessful. Though 73% view CRO Oversight & Collaboration as very important, only 30% believe they are successful.

Underperforming initiatives are characterized by low achievement of milestones in enrollment, subject compliance, and site productivity. Estimates suggest that these missed milestones result in significant delays. Enrollment, for example, typically takes twice as long to complete as originally planned. Ultimately, these delays lead to costly CRO change orders. In the same ClinOps Benchmark Report, 80% of respondents reported that they regularly miss milestones and 8 in 10 also reported that a 3-month change order costs over $100,000.
3 Steps to Avoid Costly CRO Change Orders
The case of an emerging biotech company explains a better approach. This company expected to enroll 240 patients across 85 sites in 9 short months for a pivotal phase 3 study. The small ClinOps team comprised of only two employees outsourced most of this process. To meet trial timelines, the VP of ClinOps took three steps to set their CRO partnership up for success:
1. Negotiate an Upfront Quality Agreement and CRO Oversight Plan
Effective project oversight requires continuously comparing actual operations against baseline expectations. When developing plans for CRO oversight, it is critical to ask these questions that identify clear expectations around performance and actions:
- What should be measured to assess performance?
- How do we identify an issue with performance?
- Who is responsible for resolving an issue?
- What is the escalation path?
To plan for these contingencies, the VP relied on a Quality Agreement to align with her CRO on the expected level of service for each trial operation. In developing this plan, the VP drew on best practices that outline standards, expectations, and responsibilities.
Quality Agreements provide a framework for the level of CRO service that is expected. But the VP also knew that she needed a plan that defined expectations regarding how the CRO would meet their obligations, and how performance would be measured. To this end, the VP and her CRO created a CRO Oversight Plan, which includes key milestones, goals, metrics, responsibilities, and subsidiary plans that will be delivered and tracked throughout the engagement:
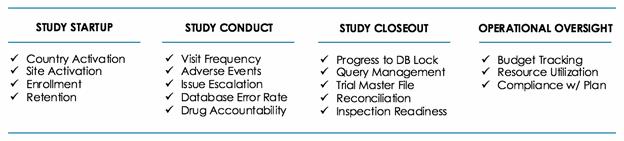
These components of an oversight plan ensure that the Sponsor and CRO are completely aligned on responsibilities and accountability through every step of the engagement.
In addition, the VP and the CRO put in place an Issue Escalation Process that starts simply and gradually increases in severity:
- Hold routine meetings: Continuously address high risk issues for alignment
- Escalate to CRO management: Align on expectations
- Remediate issues: CRO management commits to specific timeline and results
- Adjust the team: Replace CRO team members, consider adjusting resources
- Hire external oversight: Assign consultants to manage CRO performance
If this escalation path fails, the Sponsor may consider replacing the CRO, but this would only happen under rare circumstances involving disagreement and mismanagement. Once the VP and the CRO aligned on the plan and the escalation process, the teams moved to implementation.
2. Automate CRO Oversight Metrics
To be effective, both Sponsor and CRO needed accurate and timely data to track these metrics and respond to any issues before they became delays. And neither found their current system to be capable. In reviewing their current approach, the team identified three specific issues:
- Disparate data: Reconciling data from multiple sources into static spreadsheets made it challenging to identify focus areas.
- Outdated insights: The manual process of compiling reports meant that access to information was always delayed and often inaccurate.
- Disorganized task management: It was difficult to collaborate through emails, calls, and text messages that were decentralized, untraceable, and disconnected from data.
To address theses issues, the team defined system requirements. In this process, she sought advice from peers. A recent survey of 300 global ClinOps leaders identified five top requirements:
- Real-time data across systems and studies
- Unified dashboards and visualizations
- Dashboard drilldown for investigation
- Automated KPI monitoring and alerting
- Integrated analytics and collaboration
A few conversations with other ClinOps leaders confirmed these as the top requirements. The team quickly went through a streamlined Request For Proposals process and initiated implementation.
There were three critical elements to implementation that meant it took weeks, not months. First, the cloud software had existing adaptors to quickly centralize disparate data systems. Second, access to CRO data was written into their agreement and therefore included in this initial implementation phase. Finally, the staff were trained during implementation to configure the monitors and dashboards to meet their particular needs.
A few weeks later, the team was working off the same real-time data, drawing conclusions from up-to-date insights, and collaborating on escalations.
3. Manage Continuous, Real-Time Collaboration
A month into the trial, enrollment fell behind. The forecast suggested the potential for missed milestones. Both the VP and the CRO received the alert at the same time. She immediately investigated the issue by clicking into a detailed analysis of enrollment. The key analytic compared enrollment performance across each country with sites. It was immediately apparent that the U.S. was the outlier that had fallen significantly behind, while most of Europe was over-performing. She found out the U.S. was excluding a high number of patients based on a single screening criterion.
The CRO called to discuss a plan they had designed to discontinue identification of new U.S. sites, boost enrollment in Europe, and keep the clinical trial on track. Because the VP and the CRO were working with the same data, the CRO was able to be proactive and solution-oriented in managing this potential issue. Within days, the VP and the CRO had finalized a plan, including supporting data, that was ready for review by the executive sponsor. A week later, the executive approved the plan and the CRO had kicked off implementation.
Previously, the VP and CRO took two full months to identify, investigate, and address a similar issue. And the time lags often created the need for expensive change orders that poisoned the relationship. With clearly defined plans and an automated software solution, she had collaborated with her CRO to proactively manage the same issue in a week.
An Improved Partnership that Delivers Speed to Quality Results
Collectively, the team spent precious time and resources more efficiently, which, in turn, kept the trial on-time and within budget. As the extended team avoided enrollment delays, resolved outstanding issues more quickly, and reduced the number of required site visits, these benefits accrued directly to the bottom line. In this case study, clinical operations estimated over $400,000 in annual savings from more effective Sponsor-CRO collaboration. Alongside these cost savings were multiple additional benefits - including increased productivity, reduced cycle time, and mitigated risk of an FDA action.
For those facing significant challenges with clinical trial outsourcing, these three steps offer a path to improved CRO Oversight & Collaboration. Importantly, this easy-to-implement process can be in place in a few short weeks. Clinical trials following this formula will be poised for operational success, thereby increasing the chance the trial concludes on-time, within budget, and with high data quality.
