Strategic Outsourcing Often Means Combining CROs
By Kate Hammeke, director of marketing intelligence, Nice Insight
 If you’re in the business of drug development as an innovator, chances are outsourcing has become part of your strategy. Engaging outside firms to complete one or more of the steps necessary to bring a new medicine to market has been proven to add value to the process by reducing fixed costs, shortening timelines and offering access to niche expertise. With outsourcing becoming so integral to drug development strategy, the term “strategic” can be applied to almost any method of engaging outside providers, whether it is for a single element of the process or multiple steps along the way.
If you’re in the business of drug development as an innovator, chances are outsourcing has become part of your strategy. Engaging outside firms to complete one or more of the steps necessary to bring a new medicine to market has been proven to add value to the process by reducing fixed costs, shortening timelines and offering access to niche expertise. With outsourcing becoming so integral to drug development strategy, the term “strategic” can be applied to almost any method of engaging outside providers, whether it is for a single element of the process or multiple steps along the way.
There are advantages and drawbacks to each outsourcing approach, and many subcontracting relationships fall somewhere between a one-off job and a one-stop shop. The decision of whether to engage functional service providers for tactical needs or a full-service provider that can assist with a broader range of activities can only come through evaluating the needs for a specific project, coupled with the long-term needs and goals of the sponsor organization. However, understanding some benefits and disadvantages for each — in addition to learning what has worked for similar businesses — can offer insight into which practices will bring the most value to your firm.
Functional or specialty CROs tend to offer a limited range of services and have expertise in a specific therapeutic area, methodology, or service. These companies may be local or global, and they add value to sponsor organizations through access to expertise while at the same time retaining control over the process. They tend to be more flexible and have the ability to quickly respond to client needs. However, they have their limits when it comes to capabilities and capacity. Functional outsourcing can present challenges to sponsor organizations with respect to identifying the best specialty provider and in stretching resources to manage multiple providers.
Full-service CROs offer a complete range of services from preclinical development through to marketing. They enable a sponsor organization to have a single point of contact for multiple outsourced needs, which can reduce the expenses associated with identifying and vetting a CRO and the resources allocated to managing multiple vendors. It also minimizes frustrations related to miscommunication, misunderstood priorities, and delays associated with decision making. On the flip side, a full-service CRO may comprise disparate business units scattered across the globe with no guarantee of integration or communication across locations and cultures, despite standing as one brand. This can obviously nullify the inherent benefit.
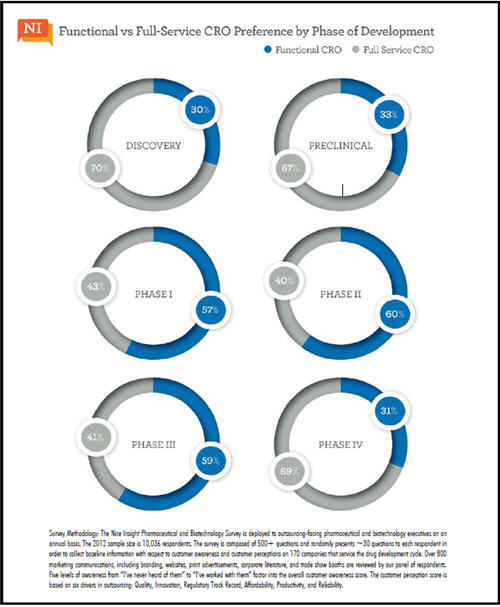
Nice Insight research has shown outsourcing preferences frequently vary across different buyer groups, and the inclination to use one outsourcing method over another is no exception. As a matter of fact, not only does the preferred method vary by sponsor category, the decision to use a functional or full-service CRO is heavily influenced by the phase of development. With the exception of Big Pharma — who display parity interest in using full-service or functional CROs — respondents show a strong preference towards engaging a full-service CRO for Discovery phase projects (70%). This is also true of preclinical phase outsourcing, where 67% of respondents indicated a preference for a full-service CRO, yet Big Pharma preferences remain even.
However, there is a distinct shift in preference when transitioning from preclinical to clinical development phases. More than half (57%) of respondents prefer a functional CRO for Phase I trials. The inclination to use a specialty CRO rather than a full-service CRO remains true for Phase II (60%) and Phase III (59%) trials, perhaps driven by small and midsize pharma companies needs, since biotech companies show an even interest in specialty and full-service CROs for Phase I-III trials. Interestingly, Big Pharma demonstrates a preference towards full-service CROs for clinical trials. This may tie back to the increasing complexity of Phase I trials, where small or midsize pharma companies seek out specialty CROs to gain access to expertise while Big Pharma companies’ internal expertise can assist in surmounting any challenges a full-service CRO encounters. The preference for full-service CROs reemerges at Phase IV (69%), and it is the only phase where a majority of respondents from each sponsor category show partiality towards full-service providers.
Deciding on whether your strategy involves the use of several functional CROs or a full-service CRO to address multiple needs won’t always be clear-cut. But learning from the experiences of your peers — as well as considering future goals and needs — can help with more informed strategic decisions to address present needs.
 If you want to learn more about the report or about how to participate, please contact Nigel Walker, managing director, or Salvatore Fazzolari, director of client services, at Nice Insight by sending an email to niceinsight.survey@thatsnice.com.
If you want to learn more about the report or about how to participate, please contact Nigel Walker, managing director, or Salvatore Fazzolari, director of client services, at Nice Insight by sending an email to niceinsight.survey@thatsnice.com.
