"Study Within A Study" Reveals Best Recruitment Strategies For Alzheimer's Trials
A conversation with Eric Siemers, MD, CMO, and Robyn Moxon, associate director of communications, Acumen Pharmaceuticals

Do you know how well your recruitment strategies work — beyond anecdotal evidence and “what we’ve always done”?
Acumen Pharmaceuticals wasn’t always so sure, but now recruitment data from its Phase 2 ALTITUDE-AD trial has shown them what truly works. The study is a Phase 2, multi-center, randomized, double-blind, placebo-controlled clinical trial designed to evaluate the efficacy and safety of sabirnetug (ACU193) infusions administered once every four weeks in slowing cognitive and functional decline as compared to placebo in participants with early Alzheimer's disease.
The company’s study recorded the origins of 2,362 screened participants across 76 sites, determining whether they came to the study via physician referral, site database, traditional ads, community engagement, family/friends, or digital ads.
The company published its findings in a poster at Clinical Trials on Alzheimer’s Disease (CTAD).
Here, Acumen CMO Eric Siemers, MD, and Associate Director of Communications Robyn Moxon, MA, discuss the findings and how they hope they’ll impact future Acumen trials and inspire the broader industry to explore their own recruitment strategies more deeply.
Clinical Leader: Take us back to the beginning. Why embark on this “study within a study” to determine the effectiveness of site-led recruitment?
Eric Siemers, MD: In our Phase 1 study of sabirnetug, we got a little bit of experience with different methods, and Robyn was comparing which seemed better. ALTITUDE is a fairly good size Phase 2 study with 542 people, so there is a lot more opportunity there. You hear a lot of anecdotes, but you don't see a lot of data. And so, fortunately, Robyn had the great idea to look more carefully and get data that will benefit us as we think about our Phase 3 study. But it also benefits the field because we've published it.
Robyn Moxon, MA: Being part of the recruitment, you always want to know if it’s successful for sites or if you're just kind of doing things and hoping for the best. And we used different strategies in our Phase 2 than our Phase 1. In Phase 1, we had a much smaller study, and we were just trying to get through it quickly. So, we did bring in some recruitment vendors and later evaluated whether those vendors were successful or not successful.
In the Phase 2 study, we focused a lot more on supporting our study sites in the recruitment efforts that they knew would work for their patient populations and their geographies rather than trying to employ one recruitment vendor to work for everybody.
That's why we pulled the data. Not a lot of other companies are sharing this kind of data. I got that feedback a lot at CTAD; they said, it's really great that you're showing this and being transparent about what's working and what's not working for this population and for this study. That's really important to me — sharing knowledge — because we're all aiming toward the same outcome anyway.
What did you intend to learn or do with the findings?
Moxon: The hope with a Phase 2 is always that it leads to a Phase 3. And a Phase 3 is usually bigger, more complicated, and maybe involves more countries. If we know what recruitment sources were more cost-effective, more timely, or had lower screen fail rates, that's going to benefit not only the company and the study progress, but it's going to put less burden on the sites and on the participants. Nobody wants to screen fail in a study. If we can try to limit that, that's always going to be helpful.
Dr. Siemers: This is an experiment that Robyn has designed, and in any kind of science experiment, one of the things you want to do is see if it replicates. I think by us publishing this information, other companies, I hope, will publish similar information, and we'll see how much consistency there is. It's part of a broader effort to use technology to make clinical trials better.
Which recruitment sources yielded the highest conversion rate — from screening through to enrollment?
Moxon: The recruitment source that had the best conversion rate was physician referrals. [See figure 2 from Acumen’s poster presentation below.] That means our study sites had a partner clinic or other physicians in their local area that referred a patient. And that had the lowest screen fail rate overall. But the site databases brought in the most participants and had the second-lowest screen fail rate. It was pretty close between those two sources as being the best for our study.
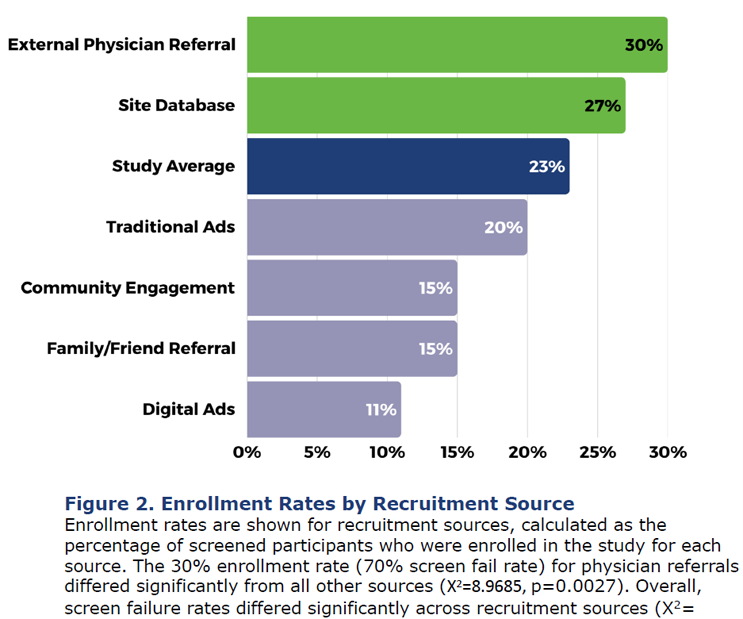
What about the various patient subgroups? Did you find any differences among them, and what were they?
Moxon: We found that our older participants and our male participants were significantly more likely to be brought in through physician referrals. And younger participants and female participants were much more likely to be brought in through digital ads.
How did the data vary across locations?
Moxon: In the U.S., we had a broader range of recruitment sources used across the study sites. It still leaned very heavily on site database as the majority, but then the rest were evenly spread out, whereas our outside the U.S. (OUS) sites were using site databases. With Germany, 98% of their screens came from a site database.
We attribute some of that to timing, because we had our U.S. sites activated prior to the OUS sites. We figure they had more time to do community outreach and put out some traditional ads, whereas European sites didn't have quite as much time and had to rely heavily on their site database.
Dr. Siemers: For context, the study was 542 people altogether. We finished recruitment in 10 months, which is pretty remarkable. We started in the U.S. and then went to Canada, and then the U.K., Germany, and Spain. It enrolled so quickly, especially in the U.S., that by the time we got to Germany and Spain, we didn't have as many slots left as we anticipated. So, the sample size is a little bit smaller in Europe. That's one thing about replication. In Germany, 98% of the people came from the database. It'd be interesting to see if that replicates in another study.
Are there any other inferences or correlations that you can make from the data?
Moxon: The Alzheimer's field is still looking at diversity, not just race and ethnicity, but in socioeconomic status, gender, sex, and education level. It would be smart to say, “Hey, physician referrals are the best because they don't have a high screen fail rate.” But you don't really want to only do one recruitment source because then your patient population might not end up being as diverse. It's important to know what is successful and what is best to put your resources toward. But it's important to diversify your recruitment in order to diversify your patient population.
And that's a big issue for the field, not just for our studies. What other people have shown — and we found in our Phase 2 study — is that even if you have a more diverse population at the beginning of the screening process for whatever reason, the cause of underrepresented people’s cognitive impairment is less likely to be Alzheimer's. They've got other causes of vascular disease, something like that. So even though you really try hard to get a diverse population, it's hard to get the actual enrolled population as diverse as you want. That's something we work on, and the whole field works on it.
Was there anything within the data that surprised you?
Moxon: Absolutely. For me, it was the digital ads having the highest screen fail rate. Being the communications person, I love social media, technology, digital ads, AI, and all these things, and seeing that they just weren't performing well in this study was kind of a bummer.
But it's an educational opportunity, because it doesn't mean that digital ads might not work. Maybe other things can be put into place — like stricter prescreening, changing the questions, or finding a way to do some stuff ahead of time to help counterbalance that screen fail rate.
Dr. Siemers: One of the things we did in the Phase 2 ALTITUDE study that we didn't do in Phase 1, because it wasn't available, is a blood test for a biomarker called PTAU-217 at the beginning of the screening procedure. And we actually screen failed a fair number of people based on that biomarker.
In the past, a lot of people would screen fail because the PET scan at the end of screening came up negative. It's a tough way to screen fail. In our case, we screen failed a lot of people based on the blood test. This overall screen failure rate wasn't so different between our Phase 1 and 2, but it was very different in terms of how they screen failed. So, even though the social media had a higher screen fail rate, you may be screen failing people in a way that's more efficient.
How will your findings impact your site selection and your patient recruitment strategies moving forward?
Dr. Siemers: The one thing, it is anecdotal, but I think it's true, is that it's not a one-size-fits-all that works at different sites. One site might say, “I've got this great database. I can always just recruit off that.” Another site may say, “I need you to send me some people to evaluate.” It just varies from site to site.
As a sponsor, you have to be flexible and not dictate too much to an individual site. On the other hand, you can't do everything. We like to have a toolbox, and the database is one tool in the toolbox. The physician referral is another tool. The social media is another tool. We can't do everything in the world, but we can give the sites some choices and let them, to some degree, pick and choose what they think will work. And then we can also share the results of our study with sites, and they can do what they want with that information.
Finally, how might sharing this data inspire or motivate other sponsor companies to also assess their recruiting strategy on their own?
Moxon: At CTAD, we had a lot of trialists come up and visit our poster, some from our study and some not from our study. And, overwhelmingly, they were not surprised by the results. They were like, “Yeah, site database, of course” and “Digital ads, they're expensive and they don't always work.” But they liked to see it, to see it in real data. It gets the conversation going. You want people to keep talking about recruitment because we want to keep focusing on it and getting patients into trials.
Dr. Siemers: I really do hope this inspires other companies to do the same sort of study and be transparent with their data, because we need to see if it replicates. I think the field can do better about using these technologies and compare and contrast how they work. Again, it may work differently in different countries, or it may not replicate. There's a big movement in clinical trials to use technology as best we can to make our trials less burdensome to the patient and the site. This is one piece to that puzzle.
From the perspective of another sponsor company, how big of a lift was this? How challenging was this as an add-on to an existing study?
Moxon: It really wasn't that much more work. We learned so much in the Phase 1 recruitment study that we built in more data points. In our Phase 2, we collected a lot more demographics and more specific recruitment questions. And we have a really great ClinOps team, data management team, and medical team, so everybody was just really keen to participate in reviewing and analyzing that data.
What about the patient or site perspective — how much extra work or effort did they have to put forth to get you that data that you needed?
Dr. Siemers: It is a really good question because we try not to overburden our sites as well as the patients. My understanding is this wasn't a lot of work for the sites, either. It's not a lot of information we need from the site. We just need to track it on our end.
About The Experts:
 Eric Siemers, MD, has more than 25 years’ experience overseeing clinical trials of neurodegenerative disease and joined Acumen as chief medical officer in 2018. He joined Eli Lilly and Company in 1998 and was responsible for several clinical trials for Alzheimer’s compounds, including five Phase 3 studies as well as Phase 1 and 2 studies. Prior to Lilly, Dr. Siemers founded the Indiana University Movement Disorder Clinic, where his research included Parkinson’s and Huntington’s diseases. Dr. Siemers served on the NIA/Alzheimer’s Association working group that proposed new research nomenclature for Alzheimer’s disease utilizing biomarkers and clinical symptoms. He was a founding member of the Alzheimer’s Association Research Roundtable and is on the steering committee for the Alzheimer’s Disease Neuroimaging Initiative. Dr. Siemers earned his MD with Highest Distinction from the Indiana University School of Medicine.
Eric Siemers, MD, has more than 25 years’ experience overseeing clinical trials of neurodegenerative disease and joined Acumen as chief medical officer in 2018. He joined Eli Lilly and Company in 1998 and was responsible for several clinical trials for Alzheimer’s compounds, including five Phase 3 studies as well as Phase 1 and 2 studies. Prior to Lilly, Dr. Siemers founded the Indiana University Movement Disorder Clinic, where his research included Parkinson’s and Huntington’s diseases. Dr. Siemers served on the NIA/Alzheimer’s Association working group that proposed new research nomenclature for Alzheimer’s disease utilizing biomarkers and clinical symptoms. He was a founding member of the Alzheimer’s Association Research Roundtable and is on the steering committee for the Alzheimer’s Disease Neuroimaging Initiative. Dr. Siemers earned his MD with Highest Distinction from the Indiana University School of Medicine.
 Robyn Moxon, MA, has over 10 years of experience supporting Alzheimer’s Disease research. She joined Acumen as the manager of corporate and clinical communications in 2022. In this role, she oversees all aspects of Acumen’s communications strategy and execution, including corporate branding, public relations, social media initiatives, and clinical trial recruitment. Robyn has an extensive background in developing successful communications programs in the medical technology space. Prior to Acumen, she worked at Functional Neuromodulation, where she led communications efforts focused on media relations, corporate reputation, social media campaigns, and clinical trial recruitment for a global Phase 3 Alzheimer’s study. She also worked with the clinical team to facilitate study activities, manage vendors, maintain clinical documents, and oversee regulatory and IRB submissions. Robyn holds an MA in Communications from Bethel University in St. Paul, MN.
Robyn Moxon, MA, has over 10 years of experience supporting Alzheimer’s Disease research. She joined Acumen as the manager of corporate and clinical communications in 2022. In this role, she oversees all aspects of Acumen’s communications strategy and execution, including corporate branding, public relations, social media initiatives, and clinical trial recruitment. Robyn has an extensive background in developing successful communications programs in the medical technology space. Prior to Acumen, she worked at Functional Neuromodulation, where she led communications efforts focused on media relations, corporate reputation, social media campaigns, and clinical trial recruitment for a global Phase 3 Alzheimer’s study. She also worked with the clinical team to facilitate study activities, manage vendors, maintain clinical documents, and oversee regulatory and IRB submissions. Robyn holds an MA in Communications from Bethel University in St. Paul, MN.
