The Critical Role Of Medical Affairs In Discovery Through Phase 2 Clinical Trials
By Uche Iloeje, MD, SpringWorks Therapeutics; Eileen K Sawyer, Ph.D., SwanBio Therapeutics; John Yee, MD, MPH, Sobi North America; and J. Ross Maclean, MD, Precision Value & Health

Medical affairs is often referred to as one of the three strategic pillars of biopharma, alongside development and commercial. In many biopharma companies, medical affairs can comprise multiple complementary capabilities, including medical communication, medical information, medical strategy, field medical, call center operations, and, depending on organizational structure, the health economics and outcomes research function as well as patient advocacy.
Yet a common refrain across biotech and pharmaceutical settings is, “When is the right time to engage medical affairs?” There are 11 unique medical affairs contributions that should begin in discovery, be directly connected to other cross-functional deliverables, and continue through launch to support a successful R&D program, helping bring novel treatment options to patients in need.
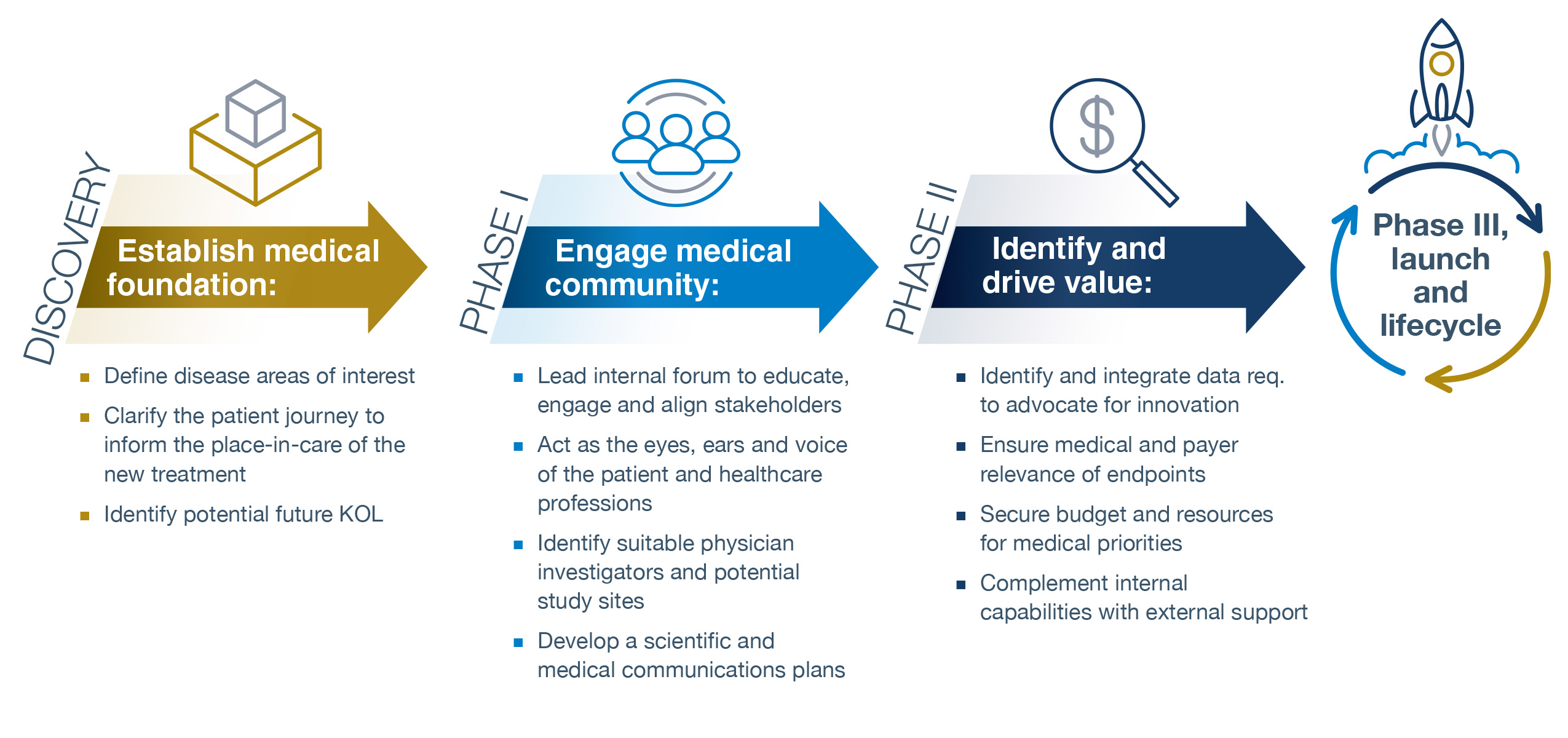
As early as discovery, medical affairs can establish the medical foundation for R&D by:
- Defining the disease areas of interest. A fundamental building block is to understand the diseases for which an emerging technology may have an application and to quantify and describe the unmet medical need (UMN) that exists and which elements of that UMN to address with the novel treatment. This may also inform future proof-of-concept studies that explore previously ignored diseases or treatment options.
- Clarifying the patient journey to inform the place-in-care of the new treatment. There must be a deep understanding of how specific diseases present in the patient and to the primary care physician and the subsequent journey through diagnosis, referral, treatment, and follow-up leading to recovery, and the many variations therein. A robust patient journey also can shape the target product profile (TPP) and the clinical feasibility of the planned trials, as well as the selection of clinical and patient-reported endpoints, in turn, linked to value demonstration.
- Identifying potential future KOLs (KOLs). The R&D program will need to be grounded in current medical practice and informed by the deep clinical knowledge of healthcare professionals from across the academic, research, and community settings. Medical personnel are ideally suited to drive the identification and outreach to potential future KOLs.
In Phase 1, medical affairs can engage with the medical community by:
- Serving as internal experts on the requirements for medical adoption of a new therapeutic. Medical affairs can facilitate cross-functional alignment by engaging peers in early R&D across the biotech or pharmaceutical firm to level-set on the disease, the different perspectives that inform the patient experience and patient outcomes, and the potential medical challenges ahead. This, in turn, will inform the medical plan and critical tactics.
- Functioning as the eyes, ears, and voice of the patient and healthcare professionals. This is done not by creating a once-and-done advisory board but rather by implementing a series of structured and unstructured engagements with the patients and their caregivers, the research community, physicians, nurses, and other healthcare professionals who care for patients in a routine practice setting. This will provide insights into what matters most and will help translate the epidemiology, clinical diagnosis, and treatment experience into the human experience (and, as previously mentioned, selecting appropriate endpoints that meet patient, regulatory, clinical, and payer expectations).
- Identifying suitable physicians, investigators, and potential study site(s). Clinical trial programs need investigators and study sites where patients can be enrolled. MA is a critical link in identifying and engaging investigators for trials, some of whom may transition into advocates for future regulatory, continuing medical education, and indication-expanding research.
- Developing a scientific and medical communications plan. Many emerging drug treatments are grounded in science that is new, highly dynamic, and has likely emerged since a prescriber’s postgraduate residency — an opportunity and a challenge for medical affairs. By understanding the prescribing physician’s knowledge base, approach to treatment, and perspective on a new treatment option, medical affairs can help the R&D organization explain a novel mechanism of action (MoA) in terms that will be meaningful to the prescriber and patient. Medical affairs can help shape greater understanding by highlighting the relevance of a drug’s MoA. In addition, the communications plan can articulate the different scientific, medical, and value messages that need to be put into the public domain and elaborate on the timing and pull-through of these messages with different external stakeholders to optimize understanding, engagement in the clinical development program, and adoption.
In Phase 2, medical affairs can identify and drive value by:
- Identifying and integrating data to advocate for innovation. Patients, caregivers, and advocates will hopefully be keen to understand and embrace a new treatment that is in development. Medical affairs can engage organizations such as medical charities, patient advocacy groups, and employer coalitions to incorporate medical innovation into real-world care appropriately. Medical affairs is ideally suited to this role given the depth of understanding of and empathy for the patient and provider experience. This is of particular importance in rare diseases where the affected population can be small, and there may be a lack of awareness of the disease and the needs of patients. Again, medical affairs can be the eyes, ears, and voice of the patient in shaping and generating the evidence during Phases 2 and 3 to support that advocacy endeavor. Medical affairs also can identify the many questions likely to arise from clinicians after drug approval, such as how a treatment may work in specific subpopulations or patients with particular comorbidities. In addition, it is critical to understand what data payers consider relevant to ensure and incorporate the generation of this information into the wider data generation plan for an asset.
- Ensuring medical and payer relevance of endpoints. Medical affairs is a key player in identifying the health outcomes (and the degree of change in those outcomes) that are meaningful to patients, health insurers, and health technology assessment (HTA) organizations. Medical affairs is uniquely positioned to understand the healthcare professional’s decision problem (DP) that is foundational to HTA clinical and economic predictive models, especially in matters related to treatment sequencing for a yet-to-be-approved drug and the evidence that will be required by the healthcare professional to inform care. During this stage, it also will be reasonable to expect that working with market access colleagues, medical affairs will jointly develop a draft set of value statements that will drive future evidence generation to bolster the value proposition for compounds in development.
- Securing the budget and resources for medical priorities. R&D resources are finite, often with a huge opportunity cost of investing in one tactic over another. Budgets reflect priorities — so a clear and early medical affairs plan that describes strategic objectives, tactics, and deliverables specific to Phases 1 and 2 will reassure senior management of the value of the investment and the timing and key performance indices to track. Such clarity, in turn, helps medical affairs set performance expectations within the organization that is critical for talent identification, development, and retention.
- Complementing internal capabilities with external support. There are two broad categories of eternal support that medical affairs can provide: (i) use of external research capabilities to extend the opportunities for an asset in development; and (ii) leveraging specialized external partners to deliver value beyond the conventional clinical trial CRO.
- By smartly leveraging the opportunities allowed by non-company-sponsored external research (investigator-initiated research and collaborative studies), medical affairs functions can greatly enhance efficiencies in R&D by generating data to support future indications, usually at a lower cost to companies than company-sponsored studies. This approach allows internal clinical development teams to focus on the core programs until they generate credible proof-of-concept data through these approaches.
- A trusted, high-quality, attentive consultant can provide medical affairs with a flexible, focused, and tailored solution for many critical services. This will allow companies to flex resources while bringing in needed expertise at the point they are needed and no sooner.
Acknowledgment
The authors are grateful for the Medical Affairs Professional Society (MAPS), which supported a live, interactive panel discussion on the topic “The critical role of medical affairs in early R&D,” out of which arose this article.
Conflict Of Interest
This article represents the views of the authors and not their employers. The authors individually report stock ownership in the firms that are their employers. There are no other conflicts of interest relevant to this article.
About The Authors:
 Uche Iloeje is senior vice president, medical affairs at SpringWorks Therapeutics, a clinical stage biopharmaceutical company based in Stamford, Connecticut. He is a Nigerian-born and -trained physician graduating from The University of Nigeria in 1987. He specialized in internal medicine at the University of Connecticut, critical care medicine at the University of Pittsburgh, and epidemiology and environmental health at Yale University. He has been in the pharmaceutical industry for over two decades, with experience in health economics and outcomes research, epidemiology, clinical development, medical affairs, and payer evidence. Dr. Iloeje has authored or co-authored over 40 peer reviewed articles in high impact journals including JAMA, Hepatology, Gastroenterology, American Journal of Epidemiology, Diabetes Obesity and Metabolism, American Journal of Cardiology, Journal of the National Cancer Institute, Journal of Clinical Oncology, and Pharmacoeconomics.
Uche Iloeje is senior vice president, medical affairs at SpringWorks Therapeutics, a clinical stage biopharmaceutical company based in Stamford, Connecticut. He is a Nigerian-born and -trained physician graduating from The University of Nigeria in 1987. He specialized in internal medicine at the University of Connecticut, critical care medicine at the University of Pittsburgh, and epidemiology and environmental health at Yale University. He has been in the pharmaceutical industry for over two decades, with experience in health economics and outcomes research, epidemiology, clinical development, medical affairs, and payer evidence. Dr. Iloeje has authored or co-authored over 40 peer reviewed articles in high impact journals including JAMA, Hepatology, Gastroenterology, American Journal of Epidemiology, Diabetes Obesity and Metabolism, American Journal of Cardiology, Journal of the National Cancer Institute, Journal of Clinical Oncology, and Pharmacoeconomics.
 Eileen Sawyer, Ph.D. currently serves as senior vice president, clinical and medical affairs, at SwanBio Therapeutics where she oversees clinical development, regulatory affairs, and medical affairs. Eileen’s professional expertise centers on drug development in rare diseases and gene therapy, and she has spent the majority of her career in small biotech. She has over 10 years’ experience in therapeutic areas ranging from inborn errors of metabolism to hematology and neurology, of which seven were in gene therapy. Prior to joining SwanBio, Eileen spent five years at uniQure where she established and led the medical affairs function in supporting the hemophilia B (Hemgenix) and Huntington disease gene therapy programs from early- to late-stage development, and three years at Alexion Pharmaceuticals where she served as the scientific communications lead for asfotase alfa (Strensiq), an enzyme replacement for a rare metabolic disease. Eileen earned her Ph.D. in neuroscience from Emory University and conducted her post-doctoral training in preclinical drug discovery at Harvard University.
Eileen Sawyer, Ph.D. currently serves as senior vice president, clinical and medical affairs, at SwanBio Therapeutics where she oversees clinical development, regulatory affairs, and medical affairs. Eileen’s professional expertise centers on drug development in rare diseases and gene therapy, and she has spent the majority of her career in small biotech. She has over 10 years’ experience in therapeutic areas ranging from inborn errors of metabolism to hematology and neurology, of which seven were in gene therapy. Prior to joining SwanBio, Eileen spent five years at uniQure where she established and led the medical affairs function in supporting the hemophilia B (Hemgenix) and Huntington disease gene therapy programs from early- to late-stage development, and three years at Alexion Pharmaceuticals where she served as the scientific communications lead for asfotase alfa (Strensiq), an enzyme replacement for a rare metabolic disease. Eileen earned her Ph.D. in neuroscience from Emory University and conducted her post-doctoral training in preclinical drug discovery at Harvard University.
 John Yee MD, MPH, most recently served as chief medical officer at Sobi North America. He has more than 25 years of leadership experience at other biotechnology, pharmaceutical, and healthcare technology companies, and at a major academic medical center. Dr. Yee has expertise in all aspects of drug development, including clinical development, medical affairs, pharmacovigilance, regulatory affairs, and patient advocacy. Prior to joining Sobi, he served in senior medical leadership roles at Genzyme Corporation, AstraZeneca Pharmaceuticals, Intarcia Therapeutics, Vertex Pharmaceuticals, and Flexion Therapeutics. Dr. Yee serves on the scientific advisory board for Comera Life Sciences and previously served as a non-executive director on the board of directors. Dr. Yee earned an MD from Harvard Medical School, an MPH in Health Care Management from the Harvard T.H. Chan School of Public Health, and an AB in History and Science from Harvard College. He completed his pediatric residency and fellowship training in rheumatology, immunology, and health services research at Boston Children’s Hospital.
John Yee MD, MPH, most recently served as chief medical officer at Sobi North America. He has more than 25 years of leadership experience at other biotechnology, pharmaceutical, and healthcare technology companies, and at a major academic medical center. Dr. Yee has expertise in all aspects of drug development, including clinical development, medical affairs, pharmacovigilance, regulatory affairs, and patient advocacy. Prior to joining Sobi, he served in senior medical leadership roles at Genzyme Corporation, AstraZeneca Pharmaceuticals, Intarcia Therapeutics, Vertex Pharmaceuticals, and Flexion Therapeutics. Dr. Yee serves on the scientific advisory board for Comera Life Sciences and previously served as a non-executive director on the board of directors. Dr. Yee earned an MD from Harvard Medical School, an MPH in Health Care Management from the Harvard T.H. Chan School of Public Health, and an AB in History and Science from Harvard College. He completed his pediatric residency and fellowship training in rheumatology, immunology, and health services research at Boston Children’s Hospital.
 Ross Maclean, MD, Ph.D., is executive vice president, head of medical affairs for PRECISIONheor and the head of medical affairs at Precision Value & Health. Dr. Maclean has over 30 years’ experience as a physician-scientist spanning primary care, occupational medicine, and health services research. He became a leader of the global health economics and outcomes research (HEOR) function for cardiovascular, metabolic, and immunology products. Dr. Maclean served as VP, HEOR, for the U.S. business across the entire Bristol Myers Squibb (BMS) portfolio, and VP, Diabetes and Cardiovascular Medical, both with BMS. Dr. Maclean also contributes to the HEOR community debate on “novel sources of value” as a complement to the conventional pharma industry approach to defining the impact of a drug on patients and society. Dr. Maclean received his doctoral degrees from the University of Aberdeen in Scotland and his MBA and master’s degree in health services and public health research, also from the University of Aberdeen. He has contributed to more than 80 publications in peer-reviewed journals.
Ross Maclean, MD, Ph.D., is executive vice president, head of medical affairs for PRECISIONheor and the head of medical affairs at Precision Value & Health. Dr. Maclean has over 30 years’ experience as a physician-scientist spanning primary care, occupational medicine, and health services research. He became a leader of the global health economics and outcomes research (HEOR) function for cardiovascular, metabolic, and immunology products. Dr. Maclean served as VP, HEOR, for the U.S. business across the entire Bristol Myers Squibb (BMS) portfolio, and VP, Diabetes and Cardiovascular Medical, both with BMS. Dr. Maclean also contributes to the HEOR community debate on “novel sources of value” as a complement to the conventional pharma industry approach to defining the impact of a drug on patients and society. Dr. Maclean received his doctoral degrees from the University of Aberdeen in Scotland and his MBA and master’s degree in health services and public health research, also from the University of Aberdeen. He has contributed to more than 80 publications in peer-reviewed journals.