The Effects Of Most Favored Nation Drug Pricing On Clinical Trial Management
By Mathini Ilancheran, senior delivery lead - research, R&D, Beroe Inc.
On May 11, 2025, President Donald Trump announced via an X post his plan to sign an executive order implementing the “Most Favored Nation” (MFN) drug pricing policy for Medicare Part B drugs, such as cancer infusions1. Effective May 12, 2025, this policy aims to align U.S. drug prices with the lowest prices available in other developed countries 0151 potentially reducing costs by 30%–80%1. As the U.S. is the largest pharmaceutical market globally, accounting for nearly 40% of global pharmaceutical sales2, such pricing reforms are likely to have downstream effects on clinical trial management (CTM).
Policy Context
The MFN policy targets government reimbursement spending under Medicare Part B, which totalled approximately $33 billion in 20212. It aims to benchmark U.S. reimbursement prices against the lowest rates paid by economically comparable nations. Originally proposed in 2020 and rescinded following legal challenges, the 2025 revival has sparked renewed debate3.
Industry organizations like PhRMA have raised concerns that MFN pricing could reduce expected returns on high-risk pharmaceutical R&D investments. Since clinical trials represent roughly 40–60% of R&D budgets, a reduction in expected revenue may lead companies to scale back or delay trial programs, particularly those involving complex protocols or niche therapeutic areas4,5. The knock-on effect could include fewer initiated trials, longer timelines, or reduced diversity in trial cohorts.
Impact On Clinical Trial Management
The MFN policy may accelerate the adoption of cost-conscious strategies across the clinical trial lifecycle. Sponsors may seek to maintain quality while optimizing for cost through approaches such as protocol simplification, site relocation to lower-cost geographies, and increased automation.
Summary of Impacts Across Clinical Trial Management
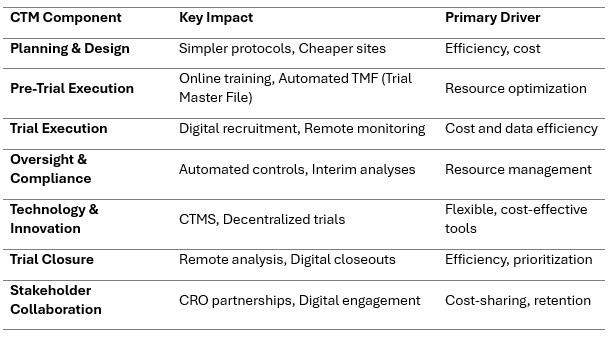
Planning & Design
To preserve margins under anticipated price ceilings, sponsors may opt for streamlined trial designs - such as using fewer clinical endpoints, shorter study duration, or smaller subject populations (e.g., ~20% fewer subjects in Phase II trials)6. While this reduces costs, it may compromise the depth of evidence collected.
Geographic reallocation to lower-cost regions such as Europe, South Asia, or Southeast Asia -where investigator site costs are typically 30%–50% lower than in the U.S- is expected to rise7. CRO negotiations may increasingly emphasize performance-based or fixed-cost pricing models to keep budgets in check8.
Pre-Trial Execution
Sponsors are shifting toward online training modules and automated Trial Master File (TMF) solutions to save time and costs associated with investigator onboarding and documentation9 .
A trend toward sourcing investigational products from international suppliers or contract manufacturers is emerging to reduce production costs. However, this also increases exposure to supply chain disruptions, such as delays in customs clearance, cold-chain integrity issues, and varying regulatory compliance standards10.
Trial Execution
Digital recruitment—through social media campaigns, search ads, or patient advocacy platforms—can reduce cost-per-enrolment by 10–25% compared to site-based recruitment models11. These approaches are especially useful for decentralized trials or rare disease studies.
Telemedicine and electronic patient-reported outcome (ePRO) tools enhance participant retention, while electronic data capture (EDC) systems — especially those with automated EHR-to-EDC integration — reduce data-entry errors and expedite data availability, cutting administrative burden and speeding trial workflows in a real-world hospital setting12. Risk-based monitoring (RBM) helps allocate monitoring resources where they matter most, improving efficiency while maintaining compliance13.
Oversight & Compliance
Automated quality control tools, such as like centralized data dashboards, machine learning models for anomaly detection, and real-time alerts, can substitute for frequent internal audits and reduce compliance costs14. These approaches have been shown to lower monitoring expenses by 15–30%, while improving accuracy15. Adaptive designs allowing prespecified modifications, such as early stopping for futility or efficacy and sample-size re-estimation, enhance trial flexibility and can shorten timelines while preserving statistical integrity16.
Technology & Innovation
Clinical trial management systems (CTMS) and decentralized trial technologies (e.g., virtual visits, wearables, mobile health apps) provide cost, time, and staffing flexibility. This is especially impactful in oncology, where average trial costs can exceed $60,000 per patient17. These platforms reduce dependencies on physical infrastructure and allow studies to reach diverse populations with fewer logistical constraints.
Trial Closure
The use of automated statistical pipelines and remote site closeout tools helps speed up final data cleaning, reconciliation, and documentation. This can reduce closeout time by several weeks and significantly lower associated labor costs17. Remote long-term follow-up, using electronic health records (EHRs) or claims data, further reduces the need for in-person assessments while preserving data continuity.
Stakeholder Collaboration
Sponsors may engage in risk-sharing or milestone-based partnerships with CROs to distribute trial cost pressures more equitably. The use of digital engagement tools such as patient portals or SMS-based reminders can help increase retention and improve communication with participants18. Collaboration across the sponsor-CRO-technology triad will be key to managing trials in a pricing-constrained environment.
Conclusion
The MFN pricing policy could introduce cost pressures that reshape the economics of clinical development. Streamlined protocols, geographic shifts, and increased reliance on external suppliers may help sponsors manage costs but could also introduce risks around data quality, regulatory compliance, and patient diversity.
However, by investing in digital, decentralized, and adaptive trial models, and by strengthening CRO collaborations, sponsors can mitigate these effects while maintaining trial integrity and accelerating time to market.
References:
- D. Trump, “X Post on Drug Pricing Executive Order,” X, May 11, 2025. [Online]. Available: https://x.com/realDonaldTrump/status/1790134567890123456
- USC Schaeffer Center for Health Policy & Economics, “Most-Favored Nation Drug Pricing Has Three Significant Problems,” 2025. [Online]. Available: https://schaeffer.usc.edu/research/most-favored-nation-drug-pricing-has-three-significant-problems/
- N. Florko, “Trump’s Drug Pricing Policy History,” STAT News, Oct. 4, 2024. [Online]. Available: https://www.statnews.com/2024/10/04/trump-prescription-drug-prices-policy-most-favored-nations/
- Pharmaceutical Research and Manufacturers of America (PhRMA), “Statement on MFN Model and Rebate Rule,” 2025. [Online]. Available: https://www.phrma.org/Press-Release/PhRMA-Statement-on-Most-Favored-Nation-Model-and-Finalized-Rebate-Rule
- T. J. Philipson and T. Durie, “The Evidence Base on the Impact of Price Controls on Medical Innovation,” Becker Friedman Institute, Working Paper No. 2021-108, Sept. 14, 2021. [Online]. Available: https://bfi.uchicago.edu/working-paper/the-evidence-base-on-the-impact-of-price-controls-on-medical-innovation/
- M. Hosely, “Collaborating with Research Sites: Best Practices for Site Selection and Study Startup,” Advarra, Oct. 4, 2022. [Online]. Available: https://www.advarra.com/blog/strategies-for-successful-site-selection-in-clinical-trials/
- Seuss+, “Top 5 Cost-Saving Strategies in Clinical Trial Contract Negotiations,” 2024. [Online]. Available: https://www.seuss.plus/blog/top-5-cost-saving-strategies-in-clinical-trial-contract-negotiations/
- Clinical Leader, “Measuring the Financial Impact of Remote Digital Clinical Trials,” 2025. [Online]. Available: https://www.clinicalleader.com/doc/measuring-the-financial-impact-of-remote-digital-clinical-trials-0001
- Anju Software, “Cut Costs in Clinical Trials with EDC,” June 4, 2019. [Online]. Available: https://www.anjusoftware.com/insights/eclinical/edc/cut-costs-clinical-trials/
- SupplyChainBrain, “Building Supply Chain Resilience in a Post-Pandemic World,” May 1, 2024. [Online]. Available: https://www.supplychainbrain.com/articles/41010-building-supply-chain-resilience-in-a-post-pandemic-world
- S. Treweek et al., “Strategies to Improve Recruitment to Randomised Trials,” Cochrane Database of Systematic Reviews, vol. 2, Feb. 2018. [Online]. Available: https://doi.org/10.1002/14651858.MR000013.pub6
- C. Mueller et al., “Automated electronic health record to electronic data capture (EHR‑to‑EDC) transfer reduces error and frees up sponsor resources: a feasibility study in Germany,” JMIR Med Inform., vol. 11, no. 3, Apr.–Jun. 2023, Art. e47958. [Online]. Available: https://www.jmir.org/2023/1/e47958/
- U.S. Food and Drug Administration (FDA), “Guidance for Industry: Risk-Based Monitoring of Clinical Investigations,” Aug. 2013. [Online]. Available: https://www.fda.gov/media/116754/download
- Medidata Solutions, “RBM and Centralized Monitoring: Cutting Costs and Increasing Accuracy,” 2022. [Online]. Available: https://www.medidata.com/en/insight/rbm-centralized-monitoring/
- Coherent Solutions, "Role of ML and AI in Clinical Trials Design: Use Cases, Benefits," Insights, Apr. 2025. [Online]. Available: https://www.coherentsolutions.com/insights/role-of-ml-and-ai-in-clinical-trials-design-use-cases-benefits
- M. Ben-Eltriki et al., “Adaptive designs in clinical trials: a systematic review—Part I,” BMC Med. Res. Methodol., vol. 24, Article 229, Oct. 4, 2024. [Online]. Available: https://doi.org/10.1186/s12874-024-02272-9
- Brookings Institution, “Addressing the Trade-Off Between Lower Drug Prices and Incentives for Pharmaceutical Innovation,” Nov. 15, 2021. [Online]. Available: https://www.brookings.edu/articles/addressing-the-trade-off-between-lower-drug-prices-and-incentives-for-pharmaceutical-innovation/
- J. Bleys, E. Fleming, H. Mirman, and L. The, “CROs and biotech companies: Fine‑tuning the partnership,” McKinsey & Company, Jun. 9, 2022. [Online]. Available: https://www.mckinsey.com/industries/life-sciences/our-insights/cros-and-biotech-companies-fine-tuning-the-partnership
About The Author:
 Mathini Ilancheran is an expert in market intelligence and industry analysis, specializing in delivering strategic insights that help Fortune 500 companies make informed decisions. With a focus on global pharma, biotech, and medical devices, she brings deep expertise in value chain analysis, industry and technology trends, competitive intelligence, and strategy. Mathini has authored 35+ publications on R&D outsourcing, offering actionable perspectives that guide global enterprises in optimizing outsourcing practices, category management, and long-term planning.
Mathini Ilancheran is an expert in market intelligence and industry analysis, specializing in delivering strategic insights that help Fortune 500 companies make informed decisions. With a focus on global pharma, biotech, and medical devices, she brings deep expertise in value chain analysis, industry and technology trends, competitive intelligence, and strategy. Mathini has authored 35+ publications on R&D outsourcing, offering actionable perspectives that guide global enterprises in optimizing outsourcing practices, category management, and long-term planning.
