The 5 Principles To Guide The Future Of Randomized Clinical Trials
By Nick Medhurst, The Good Clinical Trials Collaborative

The number of clinical trials conducted across the world is expected to rise in coming years on the back of continued demand for new innovative products and medical technologies. This could be a positive trend for global healthcare, but what tools do we have to avoid a related rise in research waste?
Already an endemic problem — and one with ethical as well as financial costs — global institutions are taking major strides to tackle research waste. In May 2022, the World Health Assembly approved a new resolution aimed at improving the coordination, design, conduct, and reporting of clinical trials worldwide, while the G7 in 2021 introduced a new charter to achieve better coordination and cooperation in clinical research and drug development around the world. The ICH renovation of E6 GCP looks set to focus regulatory requirements on ensuring trials deliver reliable results, chiefly through high-quality design and execution.
Global Community Initiative To Accelerate Better Global Healthcare
While a range of guidelines for clinical trials exist, the Good Clinical Trials Collaborative was established in 2020 to outline what “good” looks like for randomized clinical trials (RCTs) and to highlight the importance of randomization in generating robust evidence. The Collaborative is a global community initiative funded by Wellcome and the Bill & Melinda Gates Foundation and led by Professor Sir Martin Landray, chief architect of the world’s largest COVID-19 treatment trial, RECOVERY.
The Collaborative brought together a multidisciplinary global group of more than 100 members. The resultant guidance, the Guidance for Good Randomized Clinical Trials, is designed to be universally relevant to RCTs of any health intervention, for any purpose, and in any setting. It is also intended to support any individual or organization involved in the life cycle of an RCT. What makes an RCT good in its design and analysis as well as in its ethical and social value, and why is this so?
Based on five underpinning principles, the Collaborative’s guidance explores the whole journey of delivering a good RCT (i.e., one that is clinically relevant, well-planned, and well-run) and advises on range of topics that have given rise to inefficiency, irrationality, waste, and failure.
So how does the Collaborative’s guidance differ from established guidelines that govern clinical trials globally today? We believe it can help raise the quality and performance of clinical trials and the way they are regulated by extending beyond describing key activities to outlining and explaining the underlying objectives of these activities and their importance.
Importantly, the Collaborative’s guidance is not a manual describing who, what, where, or how but is a compass that helps users explore why a particular approach will achieve the right outcome for a robust trial.
While the themes of the five principles may be familiar, the considerations that support the principles have a different emphasis than traditional guidelines. Primarily, they require the user to think about the context they are working in and to seek an approach that best fits the context.
The Collaborative’s guidance recognizes the importance of innovative approaches and technologies, but their deployment may sometimes be constrained by rules that reflect how things have been done and not how they could be done better. It also aims to illuminate where common practices have sometimes come to obscure the goal of an important trial activity.
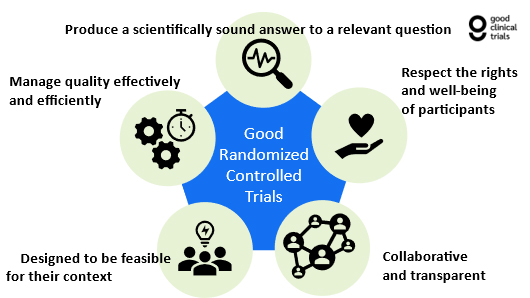
Diagram: The Five Principles of a Good RCT
The Five Principles
1. Good RCTs Are Designed To Produce Scientifically Sound Answers To Relevant Questions
The Collaborative’s guidance focuses on trials that use randomization, and the first principle covers the elements that combine to produce a sufficiently large, scientifically robust, properly randomized trial. Thousands of studies each year fail to meet that standard, so clear guidance that emphasizes the importance of, for example, completeness of follow-up and blinded assessment of outcomes, is necessary.
2. Good RCTs Respect The Rights And Well-Being Of Participants
The principle of protecting the rights, safety, and well-being of participants in clinical trials is well established but often interpreted narrowly in terms of obtaining consent and highlighting safety signals. The Collaborative’s guidance encourages users to step back and consider how the management of the safety of individual participants has important implications for the safety and well-being of all current participants, future patients, and the public in general.
3. Good RCTs Are Collaborative And Transparent
Trials are more effective when they answer questions that are genuinely relevant and meaningful to the target population, and when the answers reach those who can implement or further explore their results in a timely manner. The Collaborative’s guidance urges users to consider their own practice and responsibilities in developing partnerships and collaborating with relevant organizations and communities, including potential participant and public groups.
4. Good RCTs Are Designed To Be Feasible For Their Context
Many trials work on paper and fail in execution. Trials that work carefully to match technical rigor with practical application are more likely to succeed. The Collaborative’s guidance describes how a trial that is practicable for its context and builds on available resources, including infrastructure, facilities, skills, and expertise, can minimize waste, failure, and duplication. Aligning processes as close as possible to routine care will support the efficient and effective delivery of the trial.
5. Good RCTs Manage Quality Effectively And Efficiently
Good trials benefit from nuanced, risk-proportionate management, governance, and monitoring. The Collaborative’s guidance warns against risk-averse, prolonged, or excessive governance activities, which drive up unnecessary costs, deter trial designs of sufficient size or duration, or discourage clinicians and participants from being involved.
The guidance instead highlights the importance of focusing on monitoring, minimizing and mitigating issues that would be likely to have a meaningful impact on the well-being and safety of participants or on decision-making based on the trial results.
Read the full guidance here.
About The Author:
 Nick Medhurst is the team lead for the Good Clinical Trials Collaborative, a project committed to making it easier to do ethical and scientifically sound randomized controlled trials. Medhurst previously worked for patient representative organizations supporting people with breast cancer and cystic fibrosis (CF), and is a board member of CF Europe, a membership organization of patient representative groups, to represent and defend the interests of people with CF.
Nick Medhurst is the team lead for the Good Clinical Trials Collaborative, a project committed to making it easier to do ethical and scientifically sound randomized controlled trials. Medhurst previously worked for patient representative organizations supporting people with breast cancer and cystic fibrosis (CF), and is a board member of CF Europe, a membership organization of patient representative groups, to represent and defend the interests of people with CF.
