The Rise, Fall, And Eventual Rebound Of DCTs
By Nael Abdelsamad, MD, MBA, FACHE, CPI
When the COVID-19 pandemic hit, we all knew the research landscape would change one way or another. Patients and participants venturing out during lockdowns and risking leaving their hard-earned toilet paper stash unprotected was too crazy to fathom. Sponsors needed to find another way to keep studies open and for their trials to successfully enroll in this new world. Within a few months, you could not find an article in our space without coming across the term decentralized clinical trial or DCT. By December 2020, the Decentralized Trials & Research Alliance (DTRA) was formed to accelerate the adoption of DCTs. By then, DCTs were all the craze, and stakeholders were all for it. Studies would enroll without participants ever having to step foot in a site. Participants from every corner of the country would have access to trials like never before. Sites would slowly fade into oblivion. At least, that was the picture painted then.
Challenges And Skepticism Surrounding DCTs
Fast forward a few years, and “DCT” today has become a trigger word for many. Sites felt marginalized, believing sponsors only viewed them as responsible for implementing the studies instead of as critical stakeholders. Their frustrations with the landscape reached a tipping point, culminating with a conference intended to give them a seat at the table and save them from what they considered an untenable direction the industry was headed in. The backlash to DCTs has been brewing over the last year, with the pendulum swinging back from a vision of site-less studies utilizing a vast array of technologies to today, when sites feel they needed to protest and push a site-centric model. But why did DCTs cause such a backlash and skepticism toward a broader acceptance of the role of technology within studies? And where should the pendulum land so that patients can come out as winners at the end of this?
Though the term DCT became widely recognized during the pandemic, the first fully remote DCT was conducted almost a decade earlier (Pfizer, 2011). Although technology implementation often facilitates DCTs, digital tools and solutions are necessary even in a site-centric model. Similarly, DCTs can theoretically be implemented without necessarily relying on tech. After all, DCTs are defined as: “A clinical trial utilizing technology, processes, and services that create the opportunity to reduce or eliminate the need for participants to visit a traditional research site physically” (DTRA, n.d., emphasis added).
Sites’ apprehension with DCT workflows can be partly attributed to their negative experiences with different tech modalities. In Advarra’s 2023 Study Activation Survey, most site respondents expressed that setting up and receiving training on sponsor technology is the most challenging aspect of study startup. More than half of the sites (55%) indicated that the process of setting up and training on sponsor technology is highly burdensome, with two-thirds (67%) stating that it has become more challenging compared to just five years ago (Advarra, n.d.). In a recent survey by The Tufts Center for the Study of Drug Development, half of the respondents from clinical research sites worldwide said that they found DCT solutions more burdensome than traditional clinical trials (Maria Florez, 2024). With the barriers sites face in implementing sponsor technology, it is understandable that sites would be hesitant to embrace DCTs fully.
One of the challenges sites face with implementing technology can be attributed to sponsors' role in pushing technology from different vendors to the sites. It is common to hear sites voicing concern that sponsors are unaware of the barriers, time, and costs of implementing new technology and multiple new systems, often for the same trial. As sponsors continue to deploy more technology to their sites, there is a significant perception gap between sponsors and sites regarding the value of the technology being implemented. According to a report by Florence Healthcare in 2024, while 95% of sponsors believe that sites value their software, only 63% of sites think that the software meets their needs (Florence Healthcare, 2024). This gap in perception highlights the need for sponsors to consider the needs of sites when implementing a new system for any given trial. To bridge this gap, sponsors must work closely with sites to understand their unique needs and challenges in implementing new technology. By doing so, sponsors can ensure that the technology deployed leads to more efficient and effective clinical trials.
Perception Gap And The Hype Cycle
Overall, the adoption of DCTs across the industry follows a typical hype cycle characterized by initial excitement, inflated expectations, disillusionment, and eventual stabilization. This phenomenon is illustrated by the Gartner Hype Cycle (Figure 1), a model often used to describe the trajectory of emerging tech in various industries (Introduction to the Gartner Hype Cycle, n.d.). Initially, during the pandemic, there was a spike in interest and enthusiasm for DCTs as sponsors and other stakeholders sought innovative solutions to continue clinical trials amid lockdowns and restrictions. This led to a rapid push for technology implementation to support decentralized study workflows. For a moment, there was a prevailing belief that virtually any study could be turned into a DCT, fueling the peak of inflated expectations.
As the pandemic faded into memory, the industry began confronting the practical challenges and limitations of DCT methodologies. Expectations of the benefits from DCTs slumped, and the industry started to pivot away from the hype around DCT methodologies, shifting its focus back to the traditional site model. This disillusionment phase highlighted the complexities and barriers inherent in implementing and sustaining DCTs, prompting a reevaluation of their feasibility and effectiveness.
Expectations will likely taper off over the next few years, settling into a middle ground where DCTs and traditional site-based trials coexist and complement one another. But how do we get to the slope of enlightenment and, ultimately, the plateau of productivity?
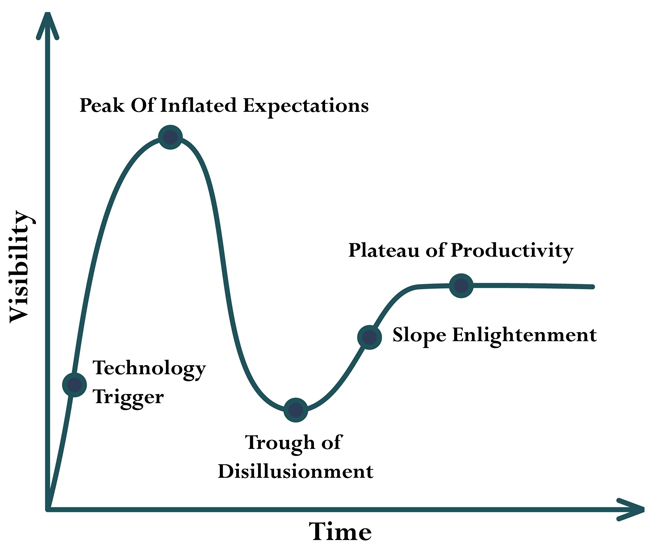
Figure illustrating Gartner Hype Cycle Curve purchased from Shutterstock by author.
Toward A Patient-Centric Approach
A patient-centric approach tailored to the target population and a technology-agnostic workflow are necessary to ensure the success of clinical trials. The study design should be mindful of the patients the study intends to recruit and should utilize the workflow and systems that facilitate this. Sites play a crucial role in enabling the success of a study, and any implemented system should take their needs into account. By implementing less burdensome technology, workflows can become more efficient, leading to better participant experiences and lower costs for sponsors (Aylin Sertkaya, 2016).
Even though DCTs have improved the accessibility of trials to our patients and participants, they should not be viewed as a one-size-fits-all solution for all studies. Instead, DCTs and traditional methods should be integrated to best serve patients' needs. To achieve long-term success and realize the potential benefits of decentralized trial methodologies, the industry also will need to manage its expectations around technology while navigating the perception gap between sponsors and sites. As the industry continues to evolve, striking the right balance between innovation and pragmatism will be essential in shaping the future of clinical research.
References:
Advarra. (n.d.). 2023 Study Activation Survey. Retrieved from https://www.advarra.com/about-advarra/news/new-clinical trial-industry-survey-reveals-increased-burdens-on-sites/
Aylin Sertkaya, H.-H. W. (2016). Key cost drivers of pharmaceutical clinical trials in the United States. Clinical Trials.
DTRA. (n.d.). DTRA Glossary. Retrieved from https://www.dtra.org/glossary
Florence Healthcare. (2024). State of Tech-Enabled Clinical Trials Report.
Introduction to the Gartner Hype Cycle. (n.d.). Retrieved from BMC: https://www.bmc.com/blogs/gartner-hype-cycle/
Maria Florez, Z. S. (2024). Quantifying Site Burden to Optimize Protocol Performance. Therapeutic Innovation & Regulatory Science.
Pfizer. (2011, June 07). Pfizer Conducts First “Virtual” Clinical Trial Allowing Patients to Participate Regardless Of Geography. Retrieved from Pfizer Conducts First “Virtual” Clinical Trial Allowing Patients to Participate Regardless Of Geography
About The Author:
 Nael Abdelsamad, MD, MBA, FACHE, CPI, is a physician and research executive with a background in trial management, clinical operations, and clinical development within academia and industry. Most recently, he served as the medical director of patient recruitment at CVS Health's Clinical Trial Services, where he led the scientific review, screening, and analysis of recruitment study opportunities. He is dual-certified by the Association of Clinical Research Professionals (Certified Principal Investigator and Certified Clinical Research Coordinator) and is a fellow of the American College of Healthcare Executives. Dr. Abdelsamad is a board member on Advarra's IBC and president of the ACRP Greater Salt Lake Chapter.
Nael Abdelsamad, MD, MBA, FACHE, CPI, is a physician and research executive with a background in trial management, clinical operations, and clinical development within academia and industry. Most recently, he served as the medical director of patient recruitment at CVS Health's Clinical Trial Services, where he led the scientific review, screening, and analysis of recruitment study opportunities. He is dual-certified by the Association of Clinical Research Professionals (Certified Principal Investigator and Certified Clinical Research Coordinator) and is a fellow of the American College of Healthcare Executives. Dr. Abdelsamad is a board member on Advarra's IBC and president of the ACRP Greater Salt Lake Chapter.
