Tracking The New Wave Of DCTs: A GlobalData Report Summary
By Life Science Connect Editorial Staff

Thanks to their ability to leverage virtual solutions that enable rapid patient recruitment and increase accessibility, decentralized clinical trials (DCTs) emerged as a solution to geographical barriers during the COVID-19 pandemic. Though global restrictions have since eased, DCTs continue to grow in popularity thanks to rapid tech development and cost-effectiveness. Sometimes referred to as virtual or direct-to-participant trials, DCTs encompass any trial with a virtual component, including data analysis, data collection, enrollment, and monitoring. In a 2022 report conducted by GlobalData using their Clinical Trials Database, researchers assessed the steady growth in DCTs from 2001 to 2022, examining a variety of factors, including indication, geography, and sponsor type. The results reveal an exciting trajectory for DCTs in the coming years.
The Shifting Landscape of DCTs
COVID-19 changed the way the drug sponsors leveraged virtual trial components. In many instances, DCTs enabled global recruitment via virtual elements like telemedicine, in-home devices, mobile healthcare, wearable devices, and virtual home visits. They also made remote data collection and observation possible. The flexibility of these technologies helped trials run as efficiently as possible throughout the pandemic. As a reflection of that, Global Data found the greatest rise in DCTs occurred from 2020-2021, with a jump from 912 to 1,293 trials.
Per Global Data’s findings, 43% of DCTs are Phase II with Phase III following, perhaps a reflection of the higher enrollment obstacles within these phases (Figure 1). While navigating the enrollment requirements of Phase II and III, DCTs help ensure accessibility for participants, and allow for efficient bulk data collection and analysis. On the other hand, early phase trials place a heavy emphasis on safety and efficacy, therefore potentially requiring more in-person monitoring of trial participants.
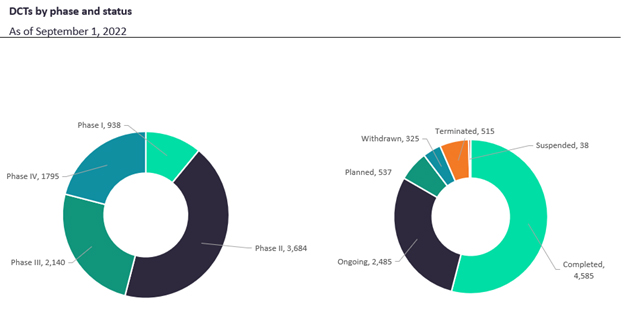
Figure 1. DCTs by phase and status
Among the possible virtual trial components, mobile healthcare is the most common, followed by trials that incorporate telemedicine, wearable devices, web-based technology, and electronic data collection. Per the World Health Organization, mobile healthcare is defined as, “medical and public health practice supported by mobile devices, such as mobile phones, patient monitoring devices, personal digital assistants, and other wireless devices.”1
As for the geographic frequency of DCTs, currently North America leads the continents in DCT usage, followed by Europe, Asia Pacific, the Middle East and Africa, and South and Central America, respectively. North America leads the charge in all DCT virtual component categories, including mobile healthcare, telemedicine, and web-based technology. Once again, Europe had the second highest usage of DCTs for all virtual component categories, apart from in-home devices which were most frequent in the Asia-Pacific region.
In terms of single-country DCTs, North America continues to lead, with nearly 1.7 times the number of single-country trials in Europe. Single country trials outnumber multinational trials in both Asia Pacific and North America, while multinational trials outnumber single-country trials in Europe, the Middle East and Africa, and South and Central America. DCTs are useful for multinational trials due to their accessibility for different populations; they make multinational recruitment easier and allow for remote patient observation and data collection.
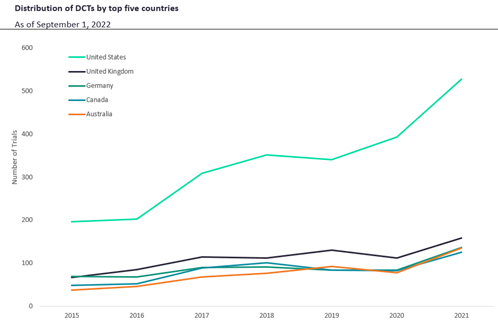
Figure 2. DCTs by leading countries
Lending significantly to the North American trend, the U.S. leads DCT usage globally and has since 2015 (Figure 2). Though notably lower than the U.S., the United Kingdom, Germany, Canada, and Australia are next highest, each with relatively similar amounts of DCT usage (Figure 2). The leading nations are all high-income countries, which makes them far more likely to conduct DCTs than middle- to lower-income countries due to the regulatory support required to adopt these approaches.
Leading Therapy Areas and Indications
As DCTs continue to trend for most indications, the central nervous system has emerged as their most prominent research therapy area, closely followed by metabolic disorders. For central nervous system DCTs, remote patient monitoring with a sensor is growing more common. Ultimately, some indications are better candidates for this than others. In oncology for example, there are fewer opportunities for home measurement trackers or sensors, leading to lower DCT numbers. Other indications may require the administration of therapeutics and/or in-person monitoring, making them ill-suited for DCTs.
Presently, type 2 diabetes is the most researched indication, followed by type 1 diabetes, COVID-19, asthma, hypertension, and chronic obstructive pulmonary disease (Figure 3). Type 1 and type 2 diabetes have become common DCT indications due to the use of glucose-measuring sensors to watch patient blood glucose levels.
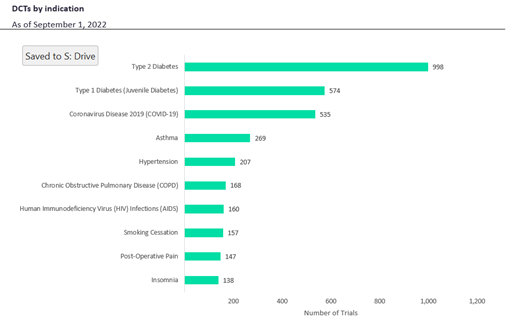
Figure 3. DCTs by indication
Present Outlook for DCTs in the Industry
As DCTs continue to grow more and more common, there are a few critical factors to consider. Since 2012, DCTs have increased from 250 to 1,291 trials; there were 1,425 projected for 2022, an all-time high. When comparing DCTs by sponsor type, industry trials achieve their endpoints at nearly 1.4 times the rate of non-industry trials. Both industry and non-industry sponsors had a similar number of trials that did not achieve endpoints. From 2012 through 2016, industry and non-industry sponsors remained close in numbers; however, from 2016 on, the number of non-industry DCTs rose above industry-sponsored DCTs (Figure 4).
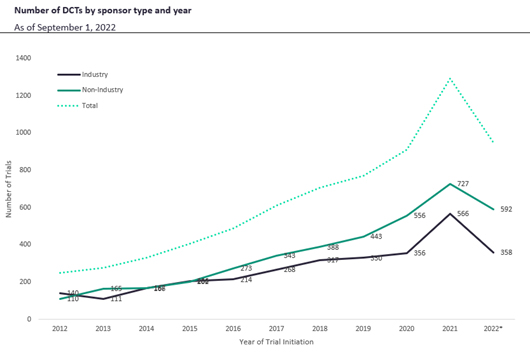
Figure 4. DCT quantity by sponsor type and year
Currently, GlaxoSmithKline (GSK) is the leading DCT industry sponsor. GSK had the most completed DCTs, followed by AstraZeneca, and Novartis. GSK’s top indications included asthma and chronic obstructive pulmonary disease. GSK also has the most suspended, terminated, and withdrawn trials. AstraZeneca has the most ongoing, followed by Novartis.
With non-industry trials, which are typically academic and/or government-funded projects, Duke led the way, closely followed by the University of Oxford, and the University of Texas MD Anderson Cancer Center. The leading indication for Duke was COVID-19, followed by amyotrophic lateral sclerosis, commonly known as ALS or Lou Gehrig’s disease. Oxford has completed the most trials, while Duke closely follows. Duke has the most ongoing and planned trials, as well as the most suspended, terminated, and withdrawn.
Currently, low accrual rate is the leading cause of trial termination. As of September 1, 2022, it was the reason for the discontinuation of 162 trials. Low accrual could be caused by a trial focusing on a specific disease or population or a lack of access to online portals. Though significantly lower, other common causes of discontinuation include lack of efficacy, financial constraints, business or strategic decisions, and adverse events. These findings may be more complex however, as not all completed trials list their endpoint status.
Looking Ahead
As DCTs continue to trend following the COVID-19 surge, they are proving to be cost-effective and efficient thanks to services like mobile healthcare, remote observation, and electronic tracking systems. Currently North America and the United States have led the DCT charge with the highest portion globally, in large part because of U.S. pharmaceutical sponsors working to streamline clinical trials. Though industry trials achieve their endpoints at a higher rate, DCTs continue to grow for both industry and non-industry sponsors, denoting an exciting movement across the clinical trial landscape.
To read the full Global Data report on DCTs, click here.
References
- Park Y. T. (2016). Emerging New Era of Mobile Health Technologies. Healthcare informatics research, 22(4), 253–254. https://doi.org/10.4258/hir.2016.22.4.253
