Underrepresentation & Overconsumption: Exploring The Gap In Global Clinical Trial Diversity
By Vladimir Misik, Ph.D., VIARES Academy for Clinical Research

In its recent guidance to the industry, the FDA expressed concern about patient diversity in industry-sponsored clinical trials (iCTs) due to a lack of enrollment of participants from underrepresented racial and ethnic populations in the United States. According to the FDA, individuals from these populations are frequently underrepresented in biomedical research despite having a disproportionate disease burden for certain diseases relative to their proportional representation in the general population.1 Without introducing corrective measures, the racial and ethnic underrepresentation in today’s iCTs in the U.S. is likely to be further amplified in coming years, as the U.S. is set to become “minority (non-Hispanic) white” by 2045.2
Therefore, the FDA urged sponsors to develop and implement operational measures that would ensure diverse clinical trial participation and improve the generation of evidence regarding safety and effectiveness across the entire population in the U.S. The FDA provided examples of measures that could include, but are not limited to, offering financial reimbursement for expenses incurred by participation in a clinical trial or study (e.g., travel or lodging), providing language access to participants with limited English language proficiency, and partnering with community-based organizations to provide support to study or trial participants.
However, the problem of insufficient representation of certain ethnic or racial groups in iCTs is not new and certainly should not be a concern to only the U.S. regulators as there are other nations/ethnicities with insufficient representation in global development of new drugs around the world. In the past, we have explored and attempted to quantify the magnitude of ethnic underrepresentation of the Middle Eastern populations (particularly Arabic).3 In this article, we examine qualifiable parameters of representation of countries in global iCTs using more recent data, introduce some new parameters providing insights into countries’ representation in iCTs, and discuss some of the medical and ethical implications.
Root Causes For Minority Underrepresentation In The U.S. And Beyond
The FDA in its recent guidance to the industry flagged the problem of underrepresentation of certain racial and ethnic populations in iCTs in the United States.1
The root causes of this phenomenon are multiple. First and foremost, we need to acknowledge the contribution of deplorable historical practices, where ethnic minorities and/or vulnerable populations were involved in research we now consider unethical.16, 17
The impact of this legacy continues to affect the inclusion of iCT subjects today in two significant ways:
Mistrust in the healthcare system persists among ethnic minorities, thus lowering their willingness to participate in CTs offered to them.18
Reverse selection bias exists among healthcare professionals, who are sensitized about potential ethical risks. Well-meaning health professionals may limit subjects’ participation in a study involving vulnerable populations under the guise of protecting these individuals from harm.19
Other factors may be at play as well, and their collective manifestation is the underrepresentation of certain socioeconomic or ethnic groups in iCTs not only in the U.S. In their 2013 paper, Noor et al. studied access to early-phase cancer trials in the U.K. as a factor of the socioeconomic statuses of patients, finding that the least deprived patients were almost twice as likely to be referred for an early-phase oncology clinical trial. Ethnicity analysis demonstrated that the non-white population was less likely to be recruited.20 In another study, Godden et al. analyzed the effect of the ethnicity of patients recruited to cancer clinical trials of all phases in one hospital trust in England, finding that non-white minorities were 30% less likely to be recruited than were white patients.21
Accessibility Further Hinders Representation
However, gaps in access to and participation in iCTs also exist on a global scale, as accessibility to iCTs for patients in several ethnically and culturally distinct global geographies lags substantially behind that of the countries in North America and Europe.3 Thus, patient diversity and adequate representation of certain ethnic groups in development of new drugs should not only be a concern to U.S. regulators but also to regulators, health professionals, and patients in countries underrepresented in global drug development.
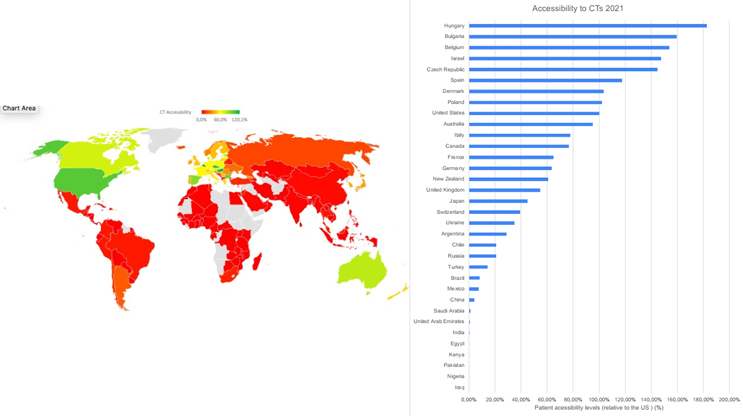
Figure 1 shows accessibility to iCTs (for definition and calculation, see Methods) for patients in countries around the world.
Figure 1. Accessibility to clinical industry clinical trials, calculated as number of clinical trial sites per 1 million in population relative to the U.S. levels (U.S. = 100%). LongTaal Clinical Trials Landscape dashboard/ linical Trials Indices.
Population-adjusted accessibility to iCTs indicates high levels of accessibility in North America and Europe, as well as Israel and Australia, with lower accessibility levels in Latin America and dropping below 5% of the U.S. levels in India, the Arabic Middle East, and Africa (with South Africa being an exception).
Low Representation, High Consumption Rates Cause Concern
While accessibility data are indicative of potential underrepresentation in iCTs, they do not reflect an important variable, i.e., consumption of pharmaceuticals. Superimposing consumption of pharmaceuticals data over iCT participation data helps us identify countries that are underrepresented in development of pharmaceuticals (i.e., in iCTs) relative to pharmaceutical consumption.
To assess these imbalances, we have introduced the Participation to Consumption Ratio (PCR) (see Appendix: Methods). Consumption of developed pharmaceuticals was expressed as global market share of pharmaceutical sales of prescription pharmaceuticals, while participation in development of novel biopharmaceutical products was expressed as a global share of active iCTs sites (iCT market share).
The PCR index has been adopted and modified from the Participation to Prevalence ratio (PPR) used by Saltzman et al. to assess proportionality of demographic representation of certain patient groups in clinical trials for cell-based therapy.22
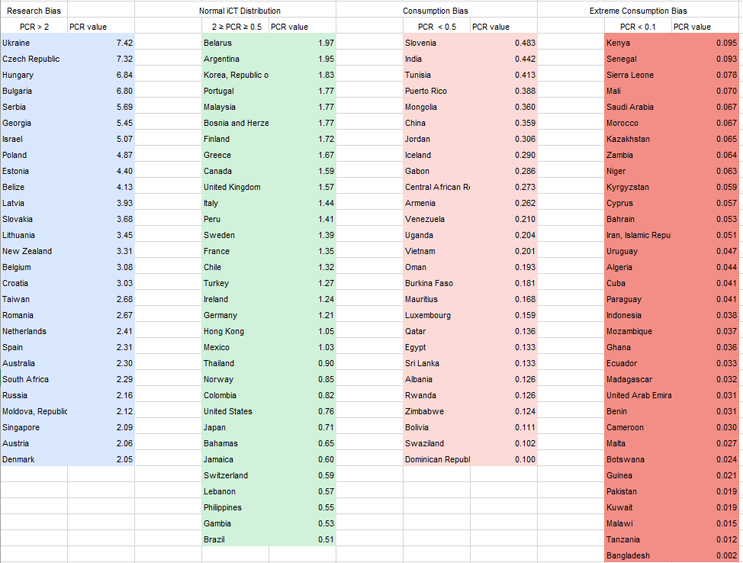
Before discussing the results, it is important to provide context for these results. We consider a “normal” or acceptable range of PCR between 0.5 and 2, while countries with a PCR index greater than 10 are, with a high degree of certainty, substantially underrepresented in the development of novel pharmaceuticals, and thus potentially consuming medications in development for which patients with similar ethnic or cultural1 profiles have not been adequately represented.
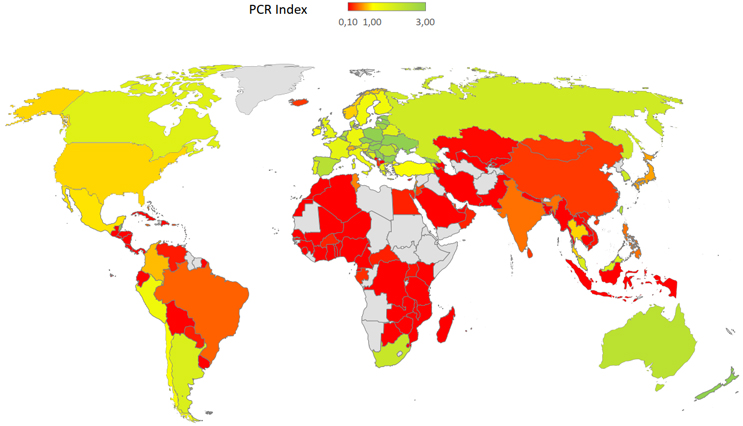
Graphical presentation of the PCR data by country are shown in Figure 2: Global heat map of PCR index by country, with darker tones of red showing the problem “out of balance” areas, with country-level details shown in Table 1.
Figure 2. Global heat map of Participation to Consumption Ratio (PCR). For definitions and methodology see Methods. LongTaal Clinical Trials Landscape dashboard, 2019 data.
Table 1. Participation in development of pharmaceuticals relative to consumption of developed pharmaceuticals (PCR index). LongTaal Clinical Trials Landscape dashboard, 2019 data.
As seen from Figure 2 and Table 1, the majority of the countries with the highest imbalances between consumption of marketed products and participation in development of new products are in Africa and the Arabic Middle East.
We recognize the following limitations of such a top-level approach to assessing representation of countries in development of novel pharmaceuticals:
Significant pricing differences exist between countries, i.e., typically lower pricing for the same compound in lower income countries.
Analysis was not done at the level of the product and/or therapeutic area.
These limitations notwithstanding, the PCR index can serve as a helpful and rapid identifier of significant imbalances. It is safe to assume that countries with a PCR index greater than 10 are substantially underrepresented in the development of novel pharmaceuticals and, thus, their residents are potentially consuming medications in development for which patients with similar ethnic or cultural profiles have not been adequately represented.
Why Underrepresentation Is A Problem
Why is insufficient ethnic representation in development of novel pharmaceuticals a problem? Let’s start with biological differences. Examples below illustrate how racial, ethnic, and cultural factors impact the safety and efficacy profile of drugs and medical devices. Such factors must therefore be taken into consideration both during products’ development stage and after their launch, including:
Skin pigmentation: Pulse oximeters function less accurately in patients with higher levels of skin pigmentation (darker skin), resulting in a risk of missing clinically important hypoxia.23 This is possibly one of the contributing factors that led to the disproportionally high COVID-19 related death rates among Black or African American, as well as Hispanic or Latino populations in the U.S.24
The effects of race and ethnicity on drug metabolism: Significant racial and ethnic variations in the pharmacokinetics, efficacy, and toxicity of drugs have been reported.25, 26
Religious and cultural practices: Drug metabolism rates could also be influenced significantly by environmental and nutritional factors such as fasting (e.g., during Ramadan). The resulting changes in drug metabolism may result in treatment failure or, conversely, in increased side effects or toxicity. Studies have shown that fasting alters drug metabolism by modulating the activity of the drug metabolizing enzymes involved.27
However, as a recent Multi-Regional Clinical Trials (MRCT) Center guidance document on patient diversity emphasized, inclusion of diverse representation in clinical trials is not simply a matter of biology but a matter of health equity, fairness, and public trust.28
3 Ways To Address The Diversity Gap
Let me touch on a few additional factors that may play a significant role in alleviating the existing diversity gaps:
Ethnically diverse iCT workforce as enablers of patient diversity:
The lack of an adequate racial/ethnic diversity radiation oncology physician workforce in the United States has been called out as a potential contributing factor to the racial/ ethnic disparities in cancer outcomes.29 Recently the U.S. NIH identified workforce diversity as a key to expanding the reach of clinical trials and inclusion of diverse populations.30 Similarly, Bierer et al. in the MRCT Center guidance document on diversity, inclusion, and equity in clinical research identified lack of cultural competence and diverse staff (investigators, referring physicians, and site staff) as one of the structural barriers to improving patient diversity in clinical research.28
The role of technology:
Decentralized or virtual CT solutions were finally embraced by the industry during the COVID-19 pandemic and supported by processes and technologies enabling telemedicine and remote patient visits. While such technologies hold a lot of promise, the practical impact on improving patient diversity in clinical trials is yet to be determined.
EHR mining with technology-based EHR-mining solutions for clinical trials appears to be uniquely suited for the identification of trial sites with access to diverse patient populations.
Patient expense reimbursement:
The FDA in its guidance to industry specifically called out offering financial reimbursement for expenses incurred by participation in a clinical trial or study (e.g., travel or lodging) as a tool to improve patient diversity.1 If handled well, this indeed can be an effective tool. However, as a recent article points out, there is an acute lack of uniform approach, including among regulators, globally.31 As a result of these differences, some geographies and regulatory jurisdictions offer no reimbursement of (often very significant) travel expenses and/or no compensation of lost wages; alternately, the process of reimbursement of these expenses may be ineffective or slow. This naturally impacts low earners disproportionally, thus reducing their interest to participate in a clinical trial or increasing their trial drop-out rates, both adversely impacting patient diversity in iCTs.
Improving Patient Diversity Is A Global Effort
This paper illustrates another dimension of patient diversity gaps in global iCTs: patients in countries/regions around the world that appear to be underrepresented in development of novel pharmaceutical relative to consumption of marketed products are thus potentially consuming medications in development for which patients with similar ethnic or cultural profile have not been adequately represented. Africa and the Middle Easthave the most countries with the highest global imbalances. These regions, as well other countries, have significantly skewed CPR balances. This problem should be further analyzed at a product level. Based on the findings, robust solution-oriented discussions involving country regulators, health policy experts, insurance providers, and biopharma representatives should take place. Possible options include requiring a sub-study on a representative population of ethnically identical or similar patient groups as part of the product marketing authorization and/or mandatory post-launch real-world data collection allowing assessment of product safety and efficacy in local populations.
We believe that in the wake of the recent FDA guidance flagging the lack of adequate ethnic diversity in iCTs, there is an opportunity to initiate discussions around this problem globally, and particularly in the affected geographies. In this context it is important to emphasize that inclusion of diverse representation in clinical trials is not simply a matter of biology but a matter of health equity, fairness, and public trust.28It remains to be seen, however, whether the market significance of the impacted markets is such that biopharma companies as sponsors of iCTs are prepared to play a more active role at driving change.
Appendix
Methods, Data Sources, And Model Assumptions
LongTaal clinical trial informatics platform combines, processes, and enriches information downloaded from ClinicalTrials.gov4 and EUDRACT5 to be used as the primary data source for comparative benchmarking analyses shown in this paper.
Clinical Trials Market Share
Unlike the methodology used by other authors, which utilizes a number of newly submitted clinical trials sites into the registries (with considerable year-on-year changes),6,7,8,9 the methodology we developed and utilized in this paper enables determination of all active clinical trial sites in the country and has proven to be reliable source for determining iCT market share of countries as a percentage of all active iCT sites in the country relative to all active iCT sites globally.3, 10, 11, 12, 13
Accessibility Of Clinical Trials
Accessibility of industry clinical trials is defined as the number of iCT sites per 1 million in population. For comparative purposes, iCT accessibility is expressed relative to the U.S. levels (U.S. iCT accessibility level being 100%). Source of the population data was the World Bank population databank.14Participation To Consumption Ratio
Participation in iCTs on a country level was approximated as a country’s market share of global iCTs (see above). As a surrogate for the consumption of pharmaceuticals on a country level, a country’s market share of global prescription sales was used. Participation to Consumption Ratio (PCR) is a parameter introduced and coined by these authors to quantify adequacy of representation of countries’ populations in development of new pharmaceutical products relative to consumption of commercially available pharmaceutical products.
Participation to Consumption Ratio
(iCT market share is calculated as shown above, and pharmaceutical consumption market share has been calculated from15.)
References
- Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry. Draft Guidance. U.S. FDA, Rockville, MD, USA. April 2022. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations [Accessed: 2022-12-15]
- Frey FH. The U.S. will become ‘minority white’ in 2045, Census projects. Brookings; March 14, 2018. Available from: https://www.brookings.edu/blog/the-avenue/2018/03/14/the-us-will-become-minority-white-in-2045-census-projects/ [Accessed: 2022-12-14]
- Misik V, Bolecek M, Brady RV. Ethical Considerations of Industry-Sponsored Clinical Trials in the Arab Region. In: Silverman H, editor. Research Ethics in the Arab Region. Research Ethics Forum, vol 5. Springer, Cham; 2017. 161 p. https://doi.org/10.1007/978-3-319-65266-5_14
- ClinicalTrials.gov. U.S. National Library of Medicine. https://clinicaltrials.gov/
- EU Clinical Trials Register. https://www.clinicaltrialsregister.eu/
- Thiers F, Sinskey AB. Trends in the globalization of clinical trials. Nat Rev Drug Discov. 2008;7:13-14. https://doi.org/10.1038/nrd2441
- Karlberg JPE. Uninterrupted Globalization of Industry Sponsored Clinical Trials. Clin. Trial Magnifier 2009;2: 79-94.
- Karlberg JPE, The Establishment of Emerging Trial Regions, Clin. Trial Magnifier 2011;4: 7-23.
- Karlberg JPE. Globalization of Industry-Sponsored, Clinical Trials. Latest Insight on Shifts in Sites Among Regions. FDAnews Management Report. Falls Church, VA (PRWEB) February 14, 2014. https://www.prweb.com/releases/fdanews/globalizationsponsoredct/prweb11583463.htm
- Misik V, Brady RV, Bolecek M, Klech H. Current Trends in Globalization of Industry-Sponsored Clinical Trials. Applied Clinical Research, Clinical Trials & Regulatory Affairs 2014;1:56-66. DOI: https://doi.org/10.2174/2213476X01666131111191016
- Misik V, Brady RV, Bolecek M, Klech H. Recent trends in globalization of industry R&D clinical trials: are emerging markets losing their allure?, Applied Clinical Research Clinical Trials and Regulatory Affairs 2017;4:175-182. DOI: https://dx.doi.org/10.2174/2213476X04666170620101442
- Misik V, Jarosz B, Beckowski L, Czarnecka M, Dabrowski T, Drake D, Milowska K, Ziecik P, Magielski P, Szczepannik W. REPORT: Industry Clinical Trials in Poland. Possibilities to increase number and scope of trials in Poland. Publishers: INFARMA and POLCRO. December 2021; pp 1-115. Available from: https://www.infarma.pl/assets/files/2022/CT_REPORT_in_PL_ANG.pdf [Accessed: 2022-12-14]
- Misik V. The Impact Of The Russia-Ukraine War On Clinical Trials. Clinical Leader, September 2022. Available from: https://www.clinicalleader.com/doc/the-impact-of-the-russia-ukraine-war-on-clinical trials-0001#:~:text=The%20war%20launched%20by%20Russia,of%20global%20patients%20in%20those [Accessed: 2022-12-14]
- World Bank. World Development Indicators (2019). Population [Data file]. Available from: https://data.worldbank.org/indicator/SP.POP.TOTL [Accessed: 2022-12-14]
- International Federation of Pharmaceutical Manufacturers & Associations. The Pharmaceutical Industry and Global Health, Facts and Figures 2021. Available from: https://www.ifpma.org/wp-content/uploads/2021/04/IFPMA-Facts-And-Figures-2021.pdf [Accessed: 2022-12-14]
- Department of Health, Education, and Welfare. The Belmont Report. Ethical Principles and Guidelines for the Protection of Human Subjects of Research. U.S. Department of Health & Human Services 18April1979. Available from: https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html [Accessed: 2022-12-14]
- Centers for Disease Control and Prevention. The U.S. Public Health Service Syphilis Study at Tuskegee. The Tuskegee Timeline. CDC, 22April2021. Available from: https://www.cdc.gov/tuskegee/timeline.htm [Accessed: 2022-12-14]
- Scharff DP, Mathews KJ, Jackson P, Hoffsuemmer J, Martin E, Edwards D. More than Tuskegee: Understanding Mistrust about Research Participation. J Healthcare Poor Underserved. 2010;21: 879–897. https://doi.org/10.1353/hpu.0.0323
- Sutton LB, Erlen JA, Glad JM, Siminoff LA; Recruiting vulnerable populations for research: Revisiting the ethical issues, Journal of Professional Nursing, Volume 19, Issue 2, 2003, pp 106-112. https://doi.org/10.1053/jpnu.2003.16.
- Noor AM, Sarker D, Vizor S, McLennan B, Hunter S, Suder A, Moller H, Spicer JF, Papa S. Effect of Patient Socioeconomic Status on Access to Early-Phase Cancer Trials. J Clin Oncol 2013;31:224-230. https://doi.org/10.1200/jco.2012.45.0999
- Godden S, Ambler G, Pollock AM. Recruitment of minority ethnic groups into clinical cancer research trials to assess adherence to the principles of the Department of Health Research Governance Framework. J Med Ethics 2010;36:358-362. DOI: https://doi.org/10.1136/jme.2009.033845
- Saltzman RG, Jayaweera DT, Caceres LV, Tovar JA, Vidro-Casiano M, Karakeshishyan V, Soto J, Khan A, Mitrani RD, Schulman IH, Hare JM. Demographic representation in clinical trials for cell-based therapy. Contemporary Clinical Trials Communications 2021;21: 100702. https://doi.org/10.1016/j.conctc.2021.100702.
- Okunlola OE, Lipnick MS, Batchelder PB, Bernstein M, Feiner JR, Bickler PE. Pulse Oximeter Performance, Racial Inequity, and the Work Ahead. Respiratory Care 2022;67:252-257. https://doi.org/10.4187/respcare.09795
- The COVID Racial Data Tracker. COVID-19 is affecting Black, Indigenous, Latinx, and other people of color the most. The COVID Tracking Projects at the Atlantic. March 7, 2021. Available from: https://covidtracking.com/race [Accessed: 2022-12-14]
- Johnson JA. Influence of race or ethnicity on pharmacokinetics of drugs. J Pharm Sci 1997;86:1328-33. https://doi.org/10.1021/js9702168
- Phan VH, Moore MM, McLachlan AJ, Piquette-Miller M, Xu H, Clarke S. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opinion on Drug Metabolism & Toxicology 2009;5:243-257. https://doi.org/10.1517/17425250902800153
- Lammers LA, Achterbergh R, Mathot RAA, Romijn JA, The effects of fasting on drug metabolism, Expert Opin Drug Metab Toxicol. 2020;16:79-85. https://doi.org/10.1080/17425255.2020.1706728
- Bierer BE, White SA, Meloney LG, Ahmed HR, Strauss DH, Clark LT. Achieving Diversity, Inclusion, and Equity in Clinical Research. Guidance Document Released 6 August 2020. Cambridge and Boston, MA: Multi-Regional Clinical Trials Center of Brigham and Women’s Hospital and Harvard (MRCT Center). Available from: https://www.fda.gov/media/142811/download [Accessed on: December 21, 2022]
- Winkfield KM, Gabeau D. Why Workforce Diversity in Oncology Matters. Int J Radiation Oncol Biol Phys 2013;85(4):900-901. https://doi.org/10.1016/j.ijrobp.2012.11.004.
- Causey M. NIH Affirms Importance of Diversity in Clinical Trial Workforce (blog). October 19, 2021. Available from: https://acrpnet.org/2021/10/19/nih-affirms-importance-of-diversity-in-clinical trial-workforce/ [Accessed: 2022-12-14]
- Novak K. Should We Pay Patients in Clinical Trials For Their Time? Clinical Leader September 29, 2022. Available from: https://www.clinicalleader.com/doc/should-we-pay-patients-in-clinical-trials-for-their-time-0001