Unleashing The Power Of Investigator-Sponsored Research (ISR) And Investigator-Initiated Trials (IIT)
By Larry Blankstein, Blankstein Consulting Group, and Sophie Gorengaut, SuperNOVA Clinical Research, Inc.

Imagine a world where the boundaries of medical science are constantly being pushed, where new drugs and devices are not just confined to their initial applications but are expanded to new indications. Such is the exciting realm of investigator-sponsored research (ISR) and investigator-initiated trials (IIT), key drivers in the evolution of healthcare. Biotech and pharma companies have increasingly recognized the value of ISR and IIT, providing support as a strategic move to broaden the application of their current drugs and for early assessment of new investigational drugs. This partnership is a delicate dance, with both parties working harmoniously toward shared goals while acknowledging the unique differences between the investigator's research objectives and industry's regulatory approval aims.
In the complex world of ISR and IIT, the support from biotech and pharmaceutical companies often goes beyond what an investigator may receive from other funding sources. This lifeline can come in the form of additional funding, drug provision, and logistical expertise, enabling the investigator to execute their study despite limited resources.
ISR and IIT represent a collaborative journey, a partnership between investigators and industry, driven by a shared vision to bring about a healthier future.
ISR Or IIT — What's The Right Option For You?
The success of this transformative partnership hinges on both parties aligning their focus and appreciating the nuances between the research objectives of investigators and industry's regulatory approval objectives. What's the difference between IIR and ISR, and what is the right option for you?
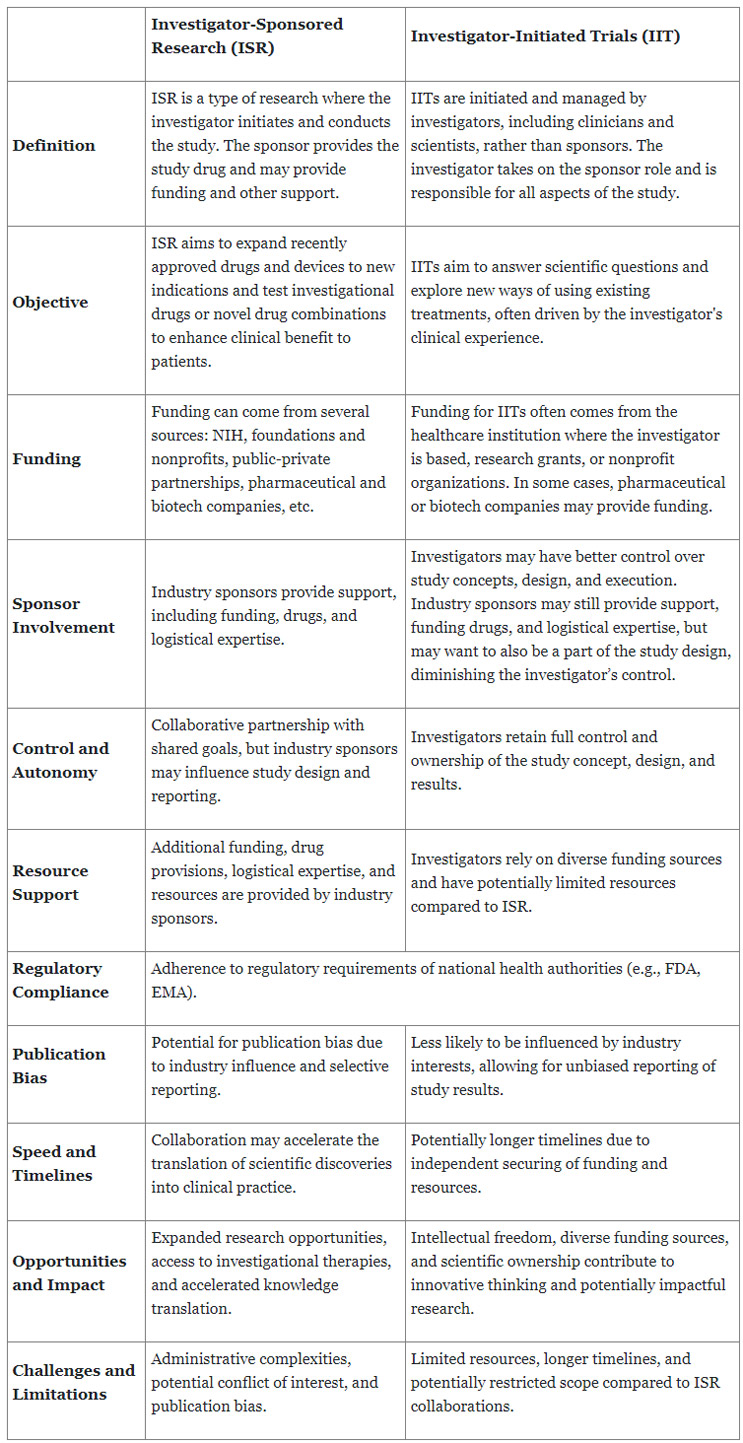
Opportunities And Challenges Of ISR And IIT
Biotech and pharma companies have embraced the ISR and IIT collaboration to enhance their drug development capabilities for the following reasons:
- Access to Investigators’ Expertise: Investigators are often well-established academic researchers or clinicians with specialized expertise in a particular disease area or therapeutic modality. Collaborating with them allows companies to access their expertise and patients while gaining valuable insights into disease mechanisms and potential new drug targets.
- Reduced Development Cost: Developing a new drug from discovery to market approval is expensive and resource-intensive. Even taking a current drug and testing it in a new modality can be costly. An ISR or IIT enables companies to share some of the financial burdens of early clinical development and get an early read on whether their new drug has benefits for patients. Other funding sources for investigator-sponsored trials can help offset costs for the industry partner.
- Patient Access: Investigators often have established patient populations and clinical research infrastructures, which can expedite patient recruitment and enrollment in clinical trials. Such access can lead to faster generation of clinical data and potentially speed up the overall drug development process. Faster data generation is undoubtedly true in rare diseases.
- Diversification of Pipeline: Collaborating with investigators allows companies to diversify their drug development pipeline by exploring new therapeutic indications or novel drug combinations. Investigator-sponsored trials may investigate drug candidates in patient populations or disease areas that the pharmaceutical company had not previously considered or don’t have the resources to pursue, thereby expanding the potential use of their drug to unmet patient populations.
Although ISR and IIT industry partnership can provide industry a cost-effective vehicle to develop novel treatments, they do come with challenges that require a collaborative approach to achieve success:
- Protocol Design/Study Objective: The primary objective of the IIT or ISR IND study may be to explore a specific hypothesis or research a question of interest to the researcher related to the investigational drug and to generate data to support additional funding. Sometimes, the investigator's and sponsor's objectives may diverge, as some sponsors may want to focus on efficacy, biomarker, or safety parameters for regulatory approval.
- Resources and Expertise: IIT may have limited resources on their research team with expertise in data management, data analysis, pharmacology, biostatistics, regulatory affairs, etc. This expertise may reside in a centralized university function that serves the entire university research community, which may not be available on a timely basis. In addition, they may not be fully aware of all the FDA requirements for data structure, capture, and analysis.
- Scope of Analysis: Investigator IND studies may collect a lot of safety, efficacy, patient-reported outcome data, etc., to be aligned with the academic research process of their institution and/or the investigator. The study may focus on the investigator’s research to fully understand the drug's biology. The funding organization requirements could also dictate some of this. The data analysis may focus on specific endpoints or outcomes relevant to the investigator's research question, which may not be aligned with the industry sponsor.
- Reporting: Investigators may prioritize publication in scientific journals to disseminate their findings and contribute to the scientific literature. The reporting of results may focus on academic and research-oriented audiences. The investigators' focus may not be on collecting all the data necessary to support a potential regulatory filing. They may also publish results in scientific journals without focusing on supporting regulatory submissions and commercial objectives.
- Data Management and Monitoring: Due to limited funding, investigators may manage study data using in-house systems or rely on external vendors for data management services that are not very robust or lack user-friendly interfaces. The investigator may conduct study monitoring for compliance and data quality, performed by in-house resources and occasionally delegated to a CRO. Industry sponsors would expect the documentation to support study oversight and regular monitoring visits by trained external monitors to ensure protocol adherence, data accuracy, and patient safety.
- Quality Assurance and Auditing: Quality assurance activities on investigator-initiated studies may be conducted internally by the investigator's institution and occur less frequently compared to industry-sponsored studies where more robust quality assurance is implemented to ensure compliance with regulatory requirements and data integrity.
Best Practices For ISR Or IIT Using White-Glove, Cost-Effective Solutions
Based on our experience working in this area, using functional service providers (FSP) or consultants rather than outsourcing to a CRO would ensure cost-effective solutions for investigators and sponsors while also providing a white-glove service. Furthermore, such a partnership will ensure success, reliable data, compliance, and efficient study execution:
- Protocol Development: The study ISR and IIT protocol development can be optimized by collaborating with a consultant with vast experience with ISRs and IITs.
- Training and Standardization: All ISR and IIT personnel involved in data collection should receive comprehensive training to ensure data consistency and adherence to the protocol. The training logs must be current and indicate each team member's specific training for their assigned task. Standard operating procedures (SOPs) can be established for many supportive study activities like patient enrollment, specific assessments, etc., and should be followed rigorously.
- Data Monitoring and Quality Control: A system for ongoing source data verification, data monitoring, and unbiased quality control of data accuracy must be implemented. Ideally, data should be reviewed regularly for completeness, accuracy, and consistency, and protocol deviations and discrepancies should be promptly identified and addressed. An outside, independent, and experienced monitor should perform the ISR or IIT monitoring.
- Electronic Data Capture (EDC) Systems: Utilizing robust, easily updated, and straightforward Part 11-compliant EDC systems can streamline data collection, minimize errors associated with manual entry, and facilitate real-time data monitoring. Data validation documentation and Case Report Forms (CRFs) completion guidelines should be developed to ensure robust data collection and accurate data entry.
- Participant Compliance and Drug Management: Implementing strategies to monitor participant compliance with study procedures is critical. A process should be in place to ensure each participant takes their medication as prescribed in the protocol participating in the study. Please also provide an ongoing study drug reconciliation throughout the study.
- Sample Collection and Management: Time-sensitive sample collection time points for pharmacokinetic (PK), biomarkers, and other key assessments must be rigorously controlled. All critical assessments that require timed sample collection should have time collection windows. Preestablished time windows in the protocol for time-sensitive study endpoints ensure more reliable and consistent data between and among study participants.
- Statistical Analysis Plan (SAP): A predefined, detailed SAP outlining the statistical methods and procedures to analyze the data should be completed before data collection initiation to minimize analysis bias.
- Analytic Methods: Key primary or secondary study endpoint analysis should have a validated assay. Small molecule analysis should comply with the FDA guidance to industry on Bioanalytical Method Validation, especially the sample re-analysis.
- Documentation and Record-Keeping: The investigator site file should be up to date and contain comprehensive and accurate documentation of all study-related activities, including data collection, analysis, and any deviations from the protocol.
Getting Ahead With ISR And IIT
ISR and IIT industry collaboration is essential to expanding the drugs currently available or under investigation, either alone or in combination, to address unmet medical needs. To accomplish this, the stakeholders must respect the goals of each party to enable the researcher to expand their research interest while executing a study that will be compliant and robust enough for the industry partner to move these innovative approaches forward to regulatory approval. There are indeed challenges to make this collaboration successful, but these can be addressed, as the outcome is well worth the effort for the researcher and industry, but most importantly for the patient.
About The Authors:
 Larry Blankstein, Ph.D., has worked hands-on and in leadership positions for over 35 years in clinical operations and project management at virtual, small, midsize, and large biotech companies as well as a CRO. As a consultant to biotechs over the past few years, he has become involved in several investigator-sponsored research projects that are focused on advancing the science and knowledge of new drug targeting, safety, and efficacy in several therapeutic areas. He understands the importance of the industry/investigator partnership in advancing drug development and the challenges this collaboration presents to the researcher and industry to ensure the goals of both parties are met.
Larry Blankstein, Ph.D., has worked hands-on and in leadership positions for over 35 years in clinical operations and project management at virtual, small, midsize, and large biotech companies as well as a CRO. As a consultant to biotechs over the past few years, he has become involved in several investigator-sponsored research projects that are focused on advancing the science and knowledge of new drug targeting, safety, and efficacy in several therapeutic areas. He understands the importance of the industry/investigator partnership in advancing drug development and the challenges this collaboration presents to the researcher and industry to ensure the goals of both parties are met.
 Sophie Gorengaut is the founder and principal consultant of SuperNOVA Clinical Research. This consultancy firm provides white-glove, agile, value-driven, cost-effective, and impactful solutions, with a network to connect clients with full-service CRO and imaging capabilities. Sophie brings about 20 years of working with pharma, biotechs, and CROs to the table. With a focus on biotech companies, Sophie embodies the core principles of SuperNOVA: dedication to excellence, nurturing relationships, and advanced expertise. Sophie's project leadership skills elevate high-performing teams, enabling her to navigate complex challenges and meet critical deadlines. With her strategic vision, she empowers biotech companies to unlock their full potential and thrive in a competitive industry.
Sophie Gorengaut is the founder and principal consultant of SuperNOVA Clinical Research. This consultancy firm provides white-glove, agile, value-driven, cost-effective, and impactful solutions, with a network to connect clients with full-service CRO and imaging capabilities. Sophie brings about 20 years of working with pharma, biotechs, and CROs to the table. With a focus on biotech companies, Sophie embodies the core principles of SuperNOVA: dedication to excellence, nurturing relationships, and advanced expertise. Sophie's project leadership skills elevate high-performing teams, enabling her to navigate complex challenges and meet critical deadlines. With her strategic vision, she empowers biotech companies to unlock their full potential and thrive in a competitive industry.
