Unlocking The Potential Of RWD: Validating Prevalence Estimates For Rare Diseases
By Shyama Ghosh, Shilpa Thakur, and Evelyn Davila, Clarivate
Real-world data (RWD) has proven to be valuable in studying rare diseases. Incorporating RWD has led to the identification of previously undetected cases and the monitoring of treatment outcomes. However, challenges such as the lack of standardized data collection and analysis methods still exist. To replicate successes, researchers should collaborate to establish standardized protocols, thereby advancing our understanding of rare diseases and improve patient outcomes. Since prompt and accurate diagnosis is crucial for the successful treatment of the rare disease Leber hereditary optical neuropathy (LHON), this comparative study of published LHON prevalence data with corresponding real-world evidence (RWE) triangulates Clarivate data assets to better understand disease pathology and gain insight into current drug use for this condition.
What Literature Tells Us About LHON — And What RWD Confirms
LHON is a rare mitochondrial disorder that primarily affects men in their 20s or 30s, causing blindness within a year due to degeneration of retinal ganglion cells. Epidemiology data sets at Clarivate show LHON prevalence rates and primary LHON mutations, morbidity, and mortality patterns for patients. A global prevalence rate of 4.3 per 100,000 population was reported in 2021, varying by country, with higher rates in northeast England and the Netherlands (Table 1). The majority of LHON patients have one of three primary mutations, and GS010, a gene therapy designed to treat LHON caused by mutations in the ND4 gene is currently in Phase 3 studies. However, the effectiveness of the therapy may vary among patients with different mutation rates, and the patient's mutational makeup may influence the timing and possibility of visual recovery.
To obtain a better understanding of LHON dynamics, disease prevalence, and drug use in the U.S., we analyzed Clarivate’s proprietary U.S. RWD product, which comprises diagnosis, pharmacy claims, and electronic health record (EHR). RWD covers 70%-80% of the insured population in all states in the U.S. All patients with a diagnosis of LHON stratified by age and gender were identified using ICD-10 and ICD-9 codes H47.2 and 377.16 annually for the period 2018-2021 using the claims data set (Figures 1, 2, & 3). They were mapped to EHR data to analyze the racial and ethnicity differences (Figure 4). We also assessed LHON treatment patterns using pharmacy and procedure claim records identified using NDC codes, as well as CPT, HCPCS, ICD-9, and ICD-10 procedure codes. We next analyzed the prescription trends in antibiotics, steroids, vitamins, and ophthalmic-intraocular pressure reducing agents (prostaglandin analogs, alpha adrenergic agonists) from 2018 to 2021 (Figure 5).
The analyses of published data and RWD provide consistent information on LHON prevalence and risk. Both data sets conclude that age and gender predict visual failure, yet an accurate forecast of disease onset in LHON carriers remains a challenge, with about 50% of male and 10% of female carriers of the primary mutations (ND4/ND6/ND1) suddenly displaying disease symptoms. Since mutational status may not significantly influence the timing of the initial visual loss, an increased focus on RWE is required to better understand what the criteria for disease onset are.
Given the promising clinical studies with GS010, the market potential is bright, and this therapy is expected to diminish LHON-related patient burden of disease. However, the therapy has a narrow window of usage after symptom onset, often limited to about three months before complete blindness may set in. In this scenario, our RWD indicate a persistent high trend of antibiotic use (amoxicillin, azithromycin, ciprofloxacin, and others) alone or in combination each year, as well as increased vitamin prescriptions (vitamins B, C, D, and others) for the period 2018-2021 (Figure 5). It is unclear how many patients are currently receiving gene therapy for LHON, even on compassionate grounds, and we support the continued use of RWD to complement ongoing clinical studies and to reduce data lags, thereby impacting the relevance and reliability of research studies.
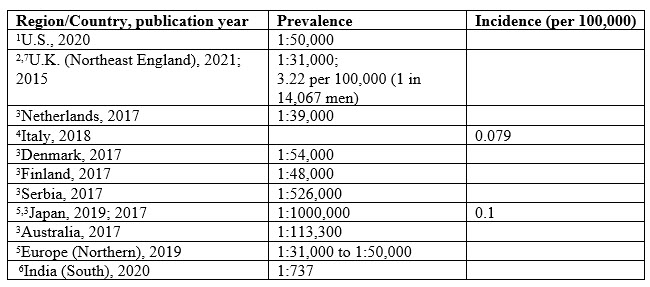
LHON’s prevalence varies between countries and regions, as reported from literature.
Table 1. LHON’s prevalence/incidence values for all ages as reported from the original study.
RWD revealed a 1.3-fold increase in the LHON prevalence per 100,000 from 2018 to 2021.
This is depicted in Figure 1. Like literature findings, RWD showed a high diagnosed prevalence of LHON in males as compared to females (Figure 2) and an increase in the diagnosed prevalence with advancing age (Figure 3).
Figure 1. Diagnosed prevalence per 100,000 of LHON in the U.S. (2018-2021)
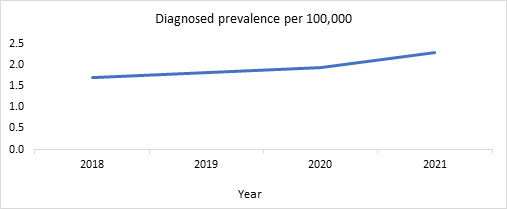
Source: Clarivate’s Real World Claims Data Product
Figure 2. Diagnosed prevalence per 100,000 of LHON by gender in the U.S. (2018-2021)
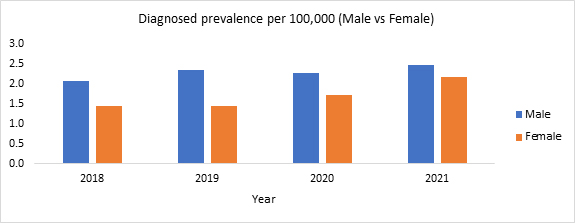
Source: Clarivate’s Real World Claims Data Product
Figure 3. Diagnosed prevalence per 100,000 of LHON by age in the U.S. (2018-2021)
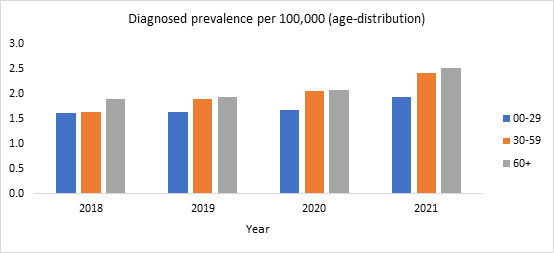
Source: Clarivate’s Real World Claims Data Product
RWD showed high diagnosed prevalent cases of LHON in non-Hispanic whites.
Figure 4. Diagnosed prevalent cases of LHON by race and ethnicity in the U.S. (2018-2021)
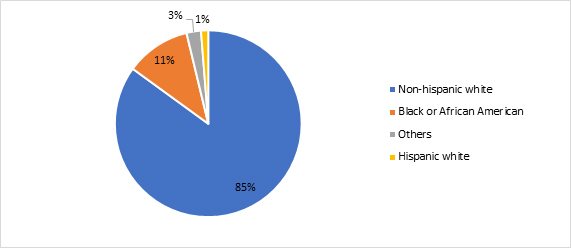
Source: Clarivate’s Real World Claims Data and Electronic Health Record Product
RWD analysis revealed that the majority of LHON patients were treated with antibiotics.
Despite the highest treatment share, antibiotics prescriptions have decreased by 7% whereas vitamin prescriptions (vitamins B, C, D, and others) increased by 78% between 2018 and 2021.
Figure 5. Drug treatment proportion of LHON in the U.S. (2018-2021)
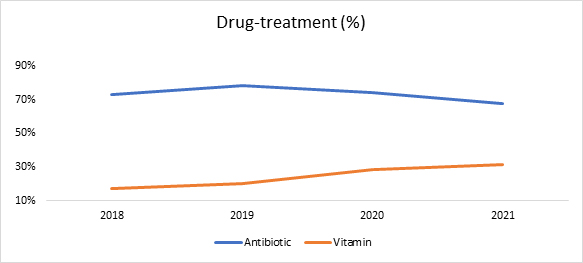
Source: Clarivate’s Real World Claims Data Product
Published studies and real-world data analyses have yielded consistent information regarding the prevalence and risk of LHON. Both data sets indicate that visual failure in LHON is predictable based on age and gender, and RWD also reveals that race/ethnicity may play a role in disease prevalence. Yet an accurate forecast of disease onset in LHON carriers remains a challenge. Research indicates that mutational status may not significantly influence the timing of the initial visual loss, therefore an increased focus on RWE for symptom onset is required.
The Limits Of And Opportunities For RWD In Researching Rare Disease
The development of gene therapy for rare diseases is promising, but without a thorough understanding of the patient population and their current diagnostic and treatment protocols, successful commercialization of these therapies may remain elusive. It is unclear how many patients are currently receiving gene therapy for LHON. Long RWD lag times may impact drug development efficiency, and we advocate for the potential of timely RWD to provide broad and recent insight into patient populations, especially regarding symptom onset.
RWD has tremendous value in studying rare diseases due to its ability to provide insights into disease prevalence, patient outcomes, and treatment effectiveness. Traditional clinical trials are often not feasible for rare diseases due to small patient populations, which makes it difficult to generate statistically significant results. RWD can help fill this gap by providing a more comprehensive understanding of the disease.
One of the specific successes of incorporating RWD in studying rare diseases is the identification of previously undetected cases. For example, a study using U.S. claims data found that the prevalence of Fabry disease was higher than previously estimated, leading to a reevaluation of screening and diagnosis guidelines. RWD also can be used to monitor treatment outcomes and identify adverse events that may not have been detected in clinical trials due to their limited sample sizes.
However, there are also some shortcomings to using RWD for studying rare diseases. One major challenge is the lack of standardized data collection and analysis methods, which can lead to inconsistencies in the quality and reliability of the data. Additionally, RWD can be biased toward certain populations or geographic regions, which can limit the generalizability of the findings.
To overcome these challenges and replicate the successes of RWD in studying rare diseases, it is important for researchers to collaborate and establish standardized protocols for data collection and analysis. It is also essential to ensure that RWD is representative of the diverse patient populations affected by rare diseases. By doing so, RWD can be a valuable tool in advancing our understanding of rare diseases and improving patient outcomes.
References:
- Sahel J et al. “Gene Therapies for the Treatment of Leber hereditary Optic Neuropathy.” International Ophthalmology Clinics; V.61; No.4; 10/2021; p195; DOI: 10.1097/IIO.0000000000000364
- Giannuzzi V et al. “Editorial: The Use of Real World Data for Regulatory Purposes in the Rare Diseases Setting.” Frontiers in Pharmacology; 13:1089033; DOI: 10.3389/fphar.2022.1089033
- Mascialino B et al. "Meta-Analysis of the Prevalence of Leber Hereditary Optic Neuropathy mtDNA Mutations in Europe." European Journal of Ophthalmology; V.22; No.3; 5/2012; p461; DOI:10.5301/ejo.5000055
- Man PYW et al. "The Epidemiology of Leber Hereditary Optic Neuropathy in the North East of England." American Journal of Human Genetics; V.72; 2003; p333
- Man PYW et al. "Leber Hereditary Optic Neuropathy." Journal of Medical Genetics; V.39; 2002; p162
- Chinnery PF et al. "The Epidemiology of Pathogenic Mitochondrial DNA Mutations." Annals of Neurology; V.48; No.2; 8/00; p188
References for Table 1:
- Filatov A et al. "Leber Hereditary Optic Neuropathy: Case Report and Literature Review." Cureus; V.12; No.4; 4/20/2020; DOI:10.7759/cureus.7745
- Sundaramurthy S et al. "Leber Hereditary Optic Neuropathy-New Insights and Old Challenges." Graefe's Archive for Clinical and Experimental Ophthalmology; V.259; No.9; 9/2021; p2461; DOI:10.1007/s00417-020-04993-1
- Ueda K et al. "Nationwide Epidemiological Survey of Leber Hereditary Optic Neuropathy in Japan." Journal of Epidemiology; V.27; No.9; 9/2017; p447; DOI:10.1016/j.je.2017.02.001
- Taruscio D et al. "The Occurrence of 275 Rare Diseases and 47 Rare Disease Groups in Italy. Results From the National Registry of Rare Diseases." International Journal of Environmental Research and Public Health; V.12; No.7; 7/12/2018; pE1470; DOI:10.3390/ijerph15071470
- Jurkute N et al. "Treatment Strategies for Leber Hereditary Optic Neuropathy." Current Opinion in Neurology; V.32; No.1; 2/2019; p99; DOI:10.1097/WCO.0000000000000646
- Gowri P et al. "A Hospital-Based Five-Year Prospective Study on the Prevalence of Leber's Hereditary Optic Neuropathy with Genetic Confirmation." Molecular Vision; 12/28/2020; p789
- Jiang P et al. "Prevalence of Mitochondrial ND4 Mutations in 1281 Han Chinese Subjects With Leber's Hereditary Optic Neuropathy." Investigative Ophthalmology and Visual Science; V.56; No.8; 7/2015; p4778; DOI:10.1167/iovs.14-16158
About The Authors:
 Shyama Ghosh has a strong interest in rare diseases, and she advocates for relevant reporting to increase awareness about such conditions. During the COVID-19 pandemic in 2020, Shyama was part of a team of Clarivate epidemiologists that created a COVID-19 medical dashboard to help vaccine manufacturers harness the large volume of information emerging from various sources. Currently, she works as a data curator for The Incidence and Prevalence Database at Clarivate.
Shyama Ghosh has a strong interest in rare diseases, and she advocates for relevant reporting to increase awareness about such conditions. During the COVID-19 pandemic in 2020, Shyama was part of a team of Clarivate epidemiologists that created a COVID-19 medical dashboard to help vaccine manufacturers harness the large volume of information emerging from various sources. Currently, she works as a data curator for The Incidence and Prevalence Database at Clarivate.
Her previous research experience includes infectious diseases, vaccine development, and molecular epidemiology at the Tropical Institute in Antwerp, Belgium. Shyama holds a Ph.D. in molecular biology from the Free University of Brussels, Belgium, and has conducted research on malaria/schistosomiasis and HPV in several African countries including Ethiopia, Cameroon, Senegal, Rwanda, and Burkina Faso.
She also has taught parasitology as a visiting professor at Jimma University in Ethiopia and the National University of Rwanda in Butare. Currently based in Antwerp, Belgium, Shyama speaks English, Hindi, Bangla, and Punjabi and is moderately fluent in Flemish and Spanish. She is a member of the Mediterranean Editors and Translators group, and her hobbies include Indian classical music, metaphysics, environmentalism, and travel.
 Shilpa Thakur joined Decision Resources Group, a part of Clarivate, in 2018 and specializes in developing epidemiological forecasts and RWD insights for multiple diseases. She received her M.P.H. from the Postgraduate Institute of Medical Education and Research with a specialization in epidemiology and biostatistics. Prior to joining Decision Resources, she monitored HIV sentinel surveillance (2016-2017) in Himachal Pradesh. She also has worked on identifying the patterns of antimicrobial resistance in India.
Shilpa Thakur joined Decision Resources Group, a part of Clarivate, in 2018 and specializes in developing epidemiological forecasts and RWD insights for multiple diseases. She received her M.P.H. from the Postgraduate Institute of Medical Education and Research with a specialization in epidemiology and biostatistics. Prior to joining Decision Resources, she monitored HIV sentinel surveillance (2016-2017) in Himachal Pradesh. She also has worked on identifying the patterns of antimicrobial resistance in India.
 Evelyn Davila, Ph.D., MPH is an epidemiologist with more than 18 years of experience in the field of observational research, epidemiology, and public health. At Clarivate, she serves as head of the epidemiology team, a large, global team of epidemiologist that supports global market sizing, forecasting, and market access decision making. She has a Ph.D. in Public Health (epidemiology specialization), master’s degree in public health, and undergraduate specializations in nutrition (major) and psychology (minor). Prior to Clarivate, she worked at Parexel as a program director for RWD-powered studies, and prior to that, she served as lead epidemiologist (team lead) at the Centers for Disease Control and Prevention (CDC), where she led the data management operations and statistical analyses of observational studies conducted in more than 20 countries.
Evelyn Davila, Ph.D., MPH is an epidemiologist with more than 18 years of experience in the field of observational research, epidemiology, and public health. At Clarivate, she serves as head of the epidemiology team, a large, global team of epidemiologist that supports global market sizing, forecasting, and market access decision making. She has a Ph.D. in Public Health (epidemiology specialization), master’s degree in public health, and undergraduate specializations in nutrition (major) and psychology (minor). Prior to Clarivate, she worked at Parexel as a program director for RWD-powered studies, and prior to that, she served as lead epidemiologist (team lead) at the Centers for Disease Control and Prevention (CDC), where she led the data management operations and statistical analyses of observational studies conducted in more than 20 countries.
