Virtual Registries: How To Bring Patient-Centricity Into Clinical Research
By Chris Isler, PA Consulting

Our rapidly increasing genetic-level disease knowledge and exponential technology advances are changing the way we manage health. But we may never fully seize these opportunities if we limit our clinical research approach to past models. Changing societal attitudes around sharing personal data and new consumer technology make innovative study designs and operations possible. This includes new possibilities in designing and operationalizing patient registries by moving to “virtual” setups.
Registries can be powerful tools to observe the course of a disease, understand variations in treatment and outcomes, examine factors influencing prognosis and quality of life, describe patterns of care, assess effectiveness, monitor safety and harm, and measure the quality of care.
Patient-Centric Registries Offer Many Benefits
Patient-centric registries can run at a fraction of the cost of traditional approaches while improving participant engagement. The approach can also capture richer, more accurate data about what affects patients’ lives and well-being most. Where traditional patient registries have complex consenting processes, extensive data capture forms, site recruitment and activation challenges, and are costly, virtual registries can minimize these common hurdles.
Leveraging digital approaches and technology, the virtual registry shifts the center of the research from the investigator to the patient and builds all research processes around the patient rather than the site. Summarizing the main differences between a site-centric and a patient-centric registry, we see that technology simplifies both key processes, such as recruitment and consenting, as well as the collection and handling of data.
The image below describes key differences between traditional and virtual registries.
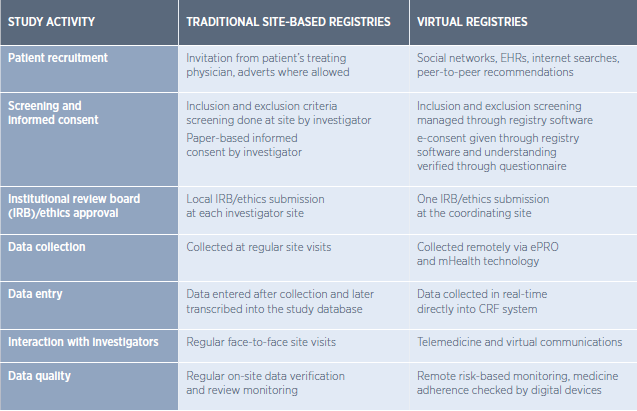
In the above, you’ll see that patient follow-up, which has been a common issue, is improved in a patient-centric registry approach. A central digital platform connects and supports all parties (patients, sponsors, investigators) in their workflows and tasks.
Hurdles To Building A Virtual Registry
There are several considerations that sponsors of virtual registries need to consider around preparing, operating, and closing a virtual registry. Key considerations include:
- Study design: Does the research question support a virtual model? E.g., if there is a strong requirement for imaging or nonstandard lab tests, a conventional model might be preferred. At the same time, if the focus is on patient reported outcomes (PROs) or novel digital endpoints, the virtual set-up will be more suitable.
- Participant engagement, retention, and safety: The more engaged the patient population is with their condition, the more they will embrace the virtual model and become advocates in recruitment and driving research. On the other hand, non-engaged patient groups might be harder to reach in a virtual model, and an investigator-led registry might deliver better results.
- Data capture and analysis: Many registries try to satisfy too many requirements — safety, market access, evidence generation — and hence capture an enormous amount of information. To make the virtual model viable, it will be critical to reduce the protocol to the key data requirements and not overburden patients and investigators.
- Participant start-up and communication: A patient-centric model requires enhanced communication, not just with investigators but also with patients. A clear communication strategy and involvement of patients in the registry governance — such as involving them in global and regional steering committees — will drive engagement and participation.
Encouraging Implementation Of Virtual Registries
By developing and implementing virtual registries in low-risk areas, such as natural history studies, we can start to create familiarity and confidence in such designs. Tackling these low-risk areas first would allow the research community to learn and share, creating best practices.
Regulators are increasingly open to and encouraging of novel registry designs. However, achieving change is a complex and difficult process; in many cases, the internal culture of research teams and the interconnected interests of different stakeholders adds to the difficulties. Focusing the case for change on cost reduction, while important, may not be the most successful approach. We have found that identifying and championing the wider benefits of such an approach will be key to winning over the range of stakeholders from the medical community, commercial life sciences R&D functions, participants, regulators, payers, and society.
As we see virtual registries and other virtual research approaches gaining traction across different disease areas and geographies, study sponsors need to consider how they can bring digital solution into their R&D units and how digital and patient-centricity capabilities can create a platform that spans across the entire pharmaceutical value chain.
 About The Author:
About The Author:
Chris Isler is a life sciences expert at PA Consulting. He has an extensive background in integrated healthcare solutions and development across Europe and the U.S. Contact him on LinkedIn at https://www.linkedin.com/in/christianisler/
