Want To Optimize Clinical Project Team Performance? Try This Formal 'Lessons Learned' Process
By Larry Blankstein, Blankstein Consulting Group, and Sophie Gorengaut, SuperNOVA Clinical Research, Inc.

Drug development is a high-risk, expensive, lengthy, and highly regulated operation where final approval is unpredictable. Under those conditions, clinical development project teams juggle many ambiguities and priorities in their journey toward approval. It is therefore essential that clinical project teams (both internal and external) have positive team dynamics and work collaboratively with open, honest, and timely communication to reach thoughtful decisions that lead to on-time, on-budget, and inspection-ready trials.
One essential ingredient that achieves that end is the implementation of a formal, structured “lessons learned” process. While some may take time to informally reflect on a trial’s ups and downs, a more calculated measure can help teams more clearly identify shortcomings and opportunities for growth. Below, describes a step-by-step process for implementing lessons learned.
5 Steps To Implementing Lessons Learned Today
Phase 1: Identify
- Approach: Gather feedback and recommendations through anonymized surveys or facilitated in-person sessions, in which honest comments are collected in a safe space.
- Why It Is Essential: The first step ensures that nuances are preserved before they are forgotten or before relevant team members transition to other assignments.
- Consequences If Not Implemented: If done after the project close-out or not at all, key lessons are missed. Opportunity for improvement is missed. Institutional knowledge is often lost when individuals transition to new roles or projects.
Phase 2: Document
- Approach: Start a formal lessons learned register using standard categories (such as ClinOps, regulatory, data management, etc.). Input raw feedback into these categories with proposed solutions.
- Why It Is Essential: Documentation in a clear, consistent format ensures that feedback is taken seriously.
- Consequences If Not Implemented: Insights may remain trapped in individual lists or silos, making it impossible for the organization to collect and share knowledge collectively. Each project may need to reinvent the wheel.
Phase 3: Analyze
- Approach: Extract meaningful strategy by performing root cause analysis (RCA) using the “5 Whys” or your other favorite technique to identify systemic and recurring issues, as well as validate best practices.
- Why It Is Essential: Analysis transforms raw data into actionable strategies and solutions. Such analysis will help distinguish between symptoms (issues) and diagnosis (the underlying cause).
- Consequences If Not Implemented: Without the RCA, you are more likely to address the wrong issue, giving a false sense of security and enabling the symptom to show up again.
Phase 4: Store
- Approach: Centralize findings in a secure, searchable lessons learned repository or corporate knowledge base equipped with keyword search capability.
- Why It Is Essential: Centralizing lessons learned sustains corporate knowledge despite staff turnover.
- Consequences If Not Implemented: Knowledge is lost to attrition or stays in silos, forcing future teams to operate without the benefit of past experiences.
Phase 5: Retrieve and Apply
- Approach: Make the review of historical lessons learned one of the required steps during the planning, risk assessment, and even the entire life cycle of each project.
- Why It Is Essential: Application of previous lessons mitigates risks that have already surfaced before, decreases implementation learning curves, and optimizes resource allocation.
- Consequences If Not Implemented: Organizations may repeat mistakes, leading to preventable delays and cost overruns.
The reference to clinical development teams above is intentionally broad. It can refer to a sponsor’s internal clinical development team, a CRO’s team on a particular project, or a combined CRO-sponsor team. Whatever the team composition, the lessons learned process remains the same. The ultimate goal is to assess any team’s health at key timepoints during the project, leading to continuous team improvement and minimizing project failures and/or delays due to poor performing/dysfunctional teams.
Goals Of Exploring Lessons Learned
- Raise constructive concerns that lead to process improvement.
- Collectively review experiences, identify successes and challenges, and translate those insights into actionable improvements.
- Open dialogue to foster respect and shared understanding, reduce miscommunication, and promote more timely and effective coordination.
- Identify workflow challenges and frustrations, resulting in actionable targeted solutions to streamline processes, reduce errors, and meet timelines.
Operating Principles Of Lessons Learned
- Focus feedback on processes and outcomes, not individuals.
- Share candid, specific, and constructive positive and negative experiences.
- Focus on solutions.
- Develop clear action items and next steps.
Key Components Of A Lessons Learned Process
A lesson learned process must have three key components: collect the knowledge, share the knowledge, and store the knowledge.
The project team should plan the timing of a lesson learned exercise at a project’s inception and incorporate it into the project timeline after achieving a major project milestone, such as completion of study startup, first participant enrolled, and/or database lock. Performing a lessons learned only at the end of the project will not contribute to continuous team improvement and success.
Collect The Knowledge
The individual conducting the lessons learned should not be a member of the project team. This will ensure unbiased, objective data collection and analysis. If this is not possible, the project manager or an independent consultant can assume this responsibility. This facilitator will develop a questionnaire to understand the team dynamics and ensure the questions reflect the issues/challenges team members believe need to be addressed. Including team member input into the lessons learned questionnaire will achieve greater team buy-in to the process. The questionnaire can capture both quantitative and narrative feedback.
If the lessons learned process includes both a sponsor and CRO team, the questionnaire can be designed to obtain sponsor and CRO feedback on all aspects of the partnership as well as each department. To maintain confidentiality, which is essential for honest team member responses and meaningful discussions, use a web-based tool like Survey Monkey for easy data collection and straightforward analysis.
Within that questionnaire, use a 1-10 scale, with 1 meaning poor and 10 meaning outstanding, to rank team leadership, problem solving, clinical operation processes, communication, collaboration with the CRO, collaboration with the sponsor, etc., to define areas for discussion.
Analyze The Knowledge
As shown in Table 1 below, the facilitator can easily collect quantitative data from the questionnaire and summarize the mean and range of all responses for each item (leadership, communications, etc.). Scores correlate to the following:
- 1-5 — needs to improve
- 6-8 — good performance
- 9-10 — high performance
The scoring segmentation will help focus the team meeting on areas requiring further discussion and action. In addition, not only is the mean of the responses important, but so is the range. If some individuals give a score of 1-3 (poor) while others give the same characteristic an 8-10 (high), deeper discussion might be required to better understand this divergence.
Table 1
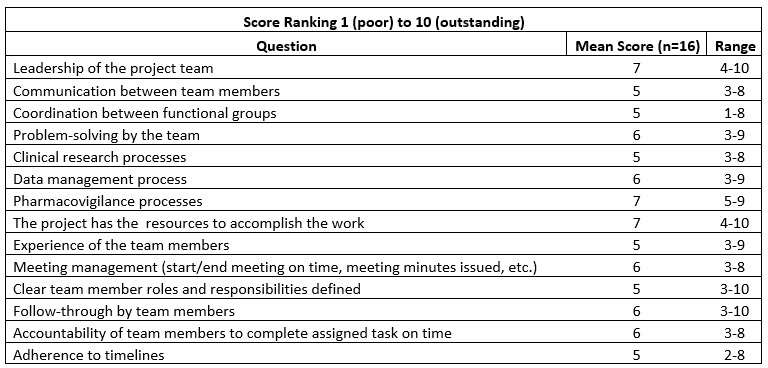
Quantitative and qualitative assessments provide the data to inform the lessons learned meeting. Qualitative questions might include:
- What went well?
- What did not go well?
- What did we do successfully and should continue doing?
- What should we never do again?
Together, qualitative and quantitative responses provide the data to:
- identify the lessons learned,
- discuss areas for corrective actions, so past mistakes are not repeated,
- improve team performance going forward, and
- identify areas of team strength that the team agrees should be maintained.
Share The Knowledge
Once data collection is complete, the facilitator will summarize the data and meet with all team members to review the data. As a team, they will reach a consensus on the lessons learned and develop an implementation plan, where required. The meeting minutes and the lessons learned data should be shared with the team and senior management. However, team managers and senior leaders should not attend the lessons learned meeting, as this could inhibit open and honest discussion. Regular review of the lessons learned and their impact on team performance should be ongoing.
Store The Knowledge
Having easy online access to historical lessons learned data can provide study teams with knowledge that could help them execute future projects. This process often requires consulting with the company’s IT department to find creative solutions for data storage and access. Consideration should be given to using keywords, drug category, or other identifiers so the information can be easily retrieved.
Top Tips For A Successful Lessons Learned Approach
- Lessons learned should be a formal process conducted at key timepoints during the study, never just at study completion, as this will not contribute to continuous team improvement. It should not be just a “check the box” exercise.
- Enhance team member buy-in by ensuring they provide input to the questionnaire.
- Keep data collection confidential to ensure honest feedback
- Discuss the data only with team members to ensure open, honest discussion and agreement on any action plans.
- Do not invite team member managers to attend the lessons learned meeting but allow them to review the output for required assistance with implementation.
- Ensure continuous review of the lessons learned during the project to assess solution implementation and to ensure the lessons learned process is not just a documentation exercise.
About The Authors:
 Larry Blankstein, Ph.D., has worked hands-on and in leadership positions for over 35 years in clinical operations and project management at virtual, small, mid-size, and large biotech companies as well as a CRO. He is keenly aware of the challenges clinical development teams face in drug development. Larry believes biotech and pharma can most effectively ensure optimal drug development by providing their teams a formal lesson learned process to optimize team performance. A process that is part of the project plans to ensure continuous team improvement and issue resolution.
Larry Blankstein, Ph.D., has worked hands-on and in leadership positions for over 35 years in clinical operations and project management at virtual, small, mid-size, and large biotech companies as well as a CRO. He is keenly aware of the challenges clinical development teams face in drug development. Larry believes biotech and pharma can most effectively ensure optimal drug development by providing their teams a formal lesson learned process to optimize team performance. A process that is part of the project plans to ensure continuous team improvement and issue resolution.
 Sophie Gorengaut is the founder and principal consultant of SuperNOVA Clinical Research, where she delivers agile clinical development solutions that balance speed, quality, and cost. Through her global network, she connects sponsors with full-service CRO and advanced imaging capabilities tailored to their specific trial needs. With nearly 20 years of experience across pharma, biotech, and CRO environments, Sophie is known for strengthening delivery models, elevating high-performing teams, and guiding complex programs through critical inflection points.
Sophie Gorengaut is the founder and principal consultant of SuperNOVA Clinical Research, where she delivers agile clinical development solutions that balance speed, quality, and cost. Through her global network, she connects sponsors with full-service CRO and advanced imaging capabilities tailored to their specific trial needs. With nearly 20 years of experience across pharma, biotech, and CRO environments, Sophie is known for strengthening delivery models, elevating high-performing teams, and guiding complex programs through critical inflection points.
