Warming Up To Hybrid Trials
By Rebecca McAvoy

The COVID-19 pandemic forced many industries to adapt to a strange new quarantined and socially distanced environment. The clinical trials space was no exception. Patients, especially very sick patients, cannot be expected to appear at trial visits as they once did. Accommodations needed to be made to enable trials to continue while maintaining patient safety – safety with respect to the trial itself and safety with respect to COVID.
The pharma industry is not known for rapid change. A recent interviewee mentioned that she feels like she is tapping into stone tablets with chisels given the slow rate of change in clinical trials. Prior to 2020, a toe was dipped into trials with online components. However, the COVID pandemic brought push to shove, and many trial sponsors and CROs had no choice but to take the plunge into hybrid trials.
In a recent survey, Industry Standard Research (ISR) asked 121 clinical trials outsourcers at sponsor companies a series of questions about their company’s use of hybrid trials. Hybrid trials were defined as the category of trials in which patients participate from home for some visits/activities rather than in a clinical setting. Forty-one percent of respondents indicated that their companies were currently using hybrid trials and another 32% say their companies have plans to start using hybrid trials in the next two years.
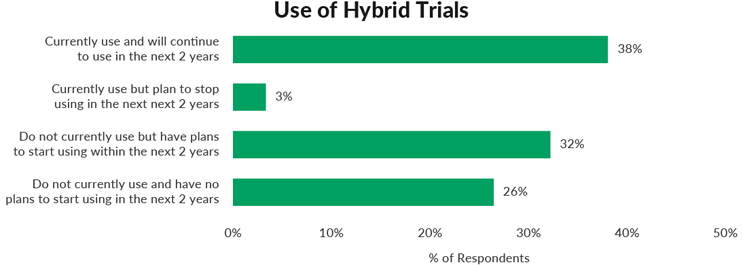
Compare this to results from a different study ISR ran the previous year – only 3% of respondents from sponsors and CROs said their companies were currently running hybrid/virtual trials and only 11% reported that their companies were even in the planning stages. The COVID pandemic in the 15 months between these surveys facilitated a large jump in the usage of hybrid trials.
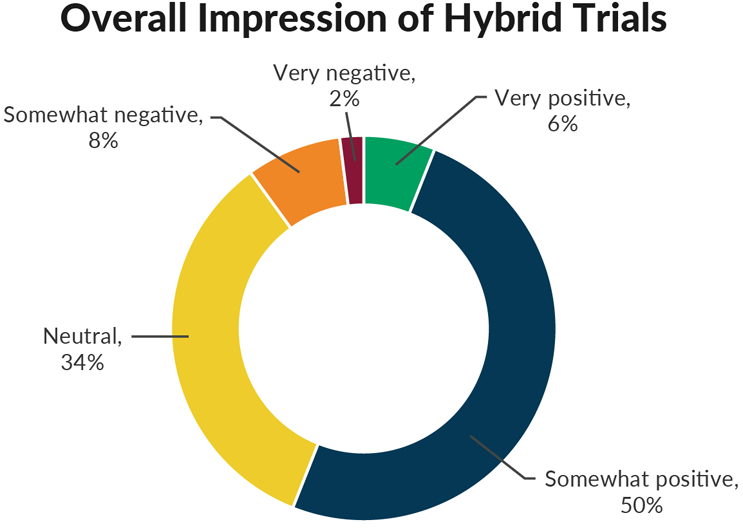
So, the use of hybrid trials has increased substantially, but how have these trials been working in practice? Among respondents whose companies are currently using hybrid trials, over half (56%) have a positive overall impression of hybrid trials. Another 34% report a neutral impression and only 10% have a negative impression.
One commonly mentioned theme for what has worked well in hybrid trials is that they enable easier participation for patients, resulting in good patient compliance.
- “Recruiting and retention of the patients has worked well as it is easier for the patients to have the study visits at home - a nurse coming to them rather than the patients going to the clinic.”
- “Patient experiences are very positive.”
- “More patient retention and compliance.”
A second positive reported outcome of hybrid trials is the speed of data collection. The online model enables fast data collection and results tabulation.
- “Reporting of data in a timely manner as data entry burden is taken off of site personnel.”
- “Speed of data analysis has been fairly good.”
- “We have had real time data.”
However, the positive feedback on what has worked well doesn’t mean that running hybrid trials is without challenges. Survey respondents also shared what has been frustrating in their experience with hybrid trials. Many responses were technology-related (technology not working properly, no available technologies to meet needs, expensive technologies), but they also ranged from extensive training needs and long setup times to challenges integrating in-person data with online data and high turnover in nursing staff.
Because hybrid trials are still quite new to the industry, there are many unknowns and concerns about running these trials. To get a sense for any discrepancy between the reality of running these trials and assumptions, we asked respondents from all companies to select up to three biggest worries or downsides regarding hybrid trials and then compared results between those whose companies are using hybrid trials and those that aren’t.
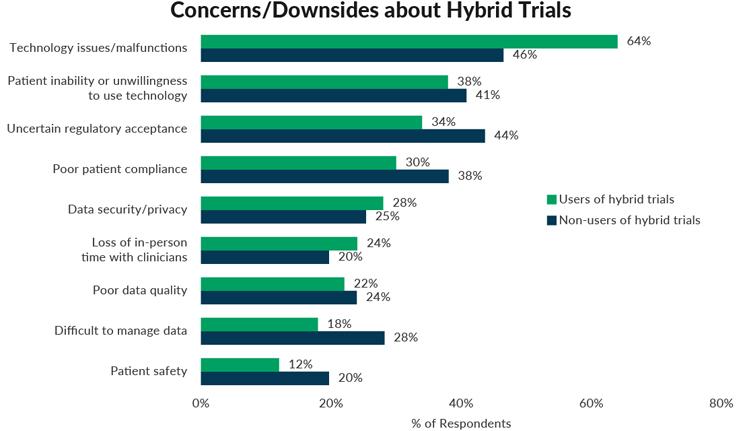
Technology issues/malfunctions proves to be the area with the biggest difference between users and non-users. Sixty-four percent of respondents whose companies are currently running hybrid trials list technology issues as a downside, compared to only 46% of respondents from companies that aren’t using hybrid trials, a difference of 18 percentage points. This indicates that respondents whose companies aren’t running hybrid trials may be underestimating the technology-related issues that arise when actually implementing hybrid trials.
On the flip side, Uncertain regulatory acceptance, Poor patient compliance, Difficult to manage data, and Patient safety prove to be more concerning to respondents whose companies don’t run hybrid trials compared to respondents whose companies do. Respondents whose companies are inexperienced with running hybrid trials may be more concerned with these potential downsides than actual experience with these trials dictates.
