What Are The Financial Barriers To Site Sustainability, Patient Experience, & Overall Trial Success?
By Jessica Knott, Society for Clinical Research Sites (SCRS)

Since its founding in 2012, the Society for Clinical Research Sites (SCRS) has advocated for rapid and accurate site payments. With her extensive background in clinical research, SCRS founder Christine Pierre had an in-depth understanding of the complexities of the industry and the need for key stakeholders to prioritize timely and accurate site payments using intuitive systems.
In 2006, Pierre hosted the inaugural Site Solutions Summit under RxTrials, a site network she established, with the goal of both educating and providing sites with a platform to voice their concerns and evolving needs. As the Summit grew from year to year, attending sites identified barriers to their success, and sponsors and CROs began to pay attention and address their feedback. The first annual Site Landscape Survey was conducted in 2008, providing insights directly from sites to the global clinical research community.
The 2010 Site Landscape Survey results indicated that 74 percent of sites wanted sponsors and CROs to pay sites more rapidly as work is completed, with monthly payments preferred.
SCRS has been highlighting sites’ need for monthly payments since its inception. SCRS tracks clinical trial site trends, with recent Site Landscape Survey data showing the following:
- 39 percent of sites were receiving monthly payment terms in 2017;
- Only 28 percent of sites signed CTAs with monthly payment terms (49 percent with quarterly payment terms);
- 77 percent of sites prefer a monthly payment schedule; and
- 60 percent of sites try to negotiate better payment terms every time, yet only 6 percent of sites are successful in doing so.
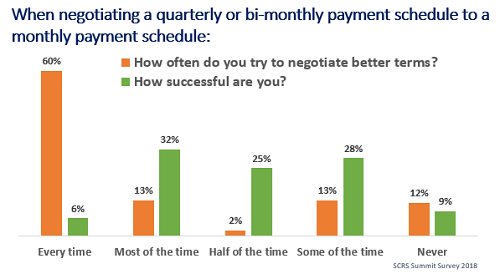
In 2016, SCRS partnered with Greenphire, a clinical trial financial solutions provider, to collect the voices of sites on clinical trial payments and patient reimbursements. The resulting white paper, published in 2017, validated SCRS’ finding that sites need more frequent payment and outlined the complexity of the processes of paying both sites and patients. Four key needed improvements were specified:
- Timely payment within 30 days;
- Electronic payment via electronic funds transfer;
- Site access to their financial information in payers’ electronic systems; and
- Automatic payment with reduced manual invoicing.
In 2018, SCRS and Greenphire conducted a deep-dive follow-up survey focusing on rapid and automated payments and the current financial barriers to site sustainability, patient experience, and overall trial success. Emerging themes from the follow-up survey clarified the need for user-friendly, intuitive data capture and payment systems along the clinical trial life cycle, with on-call support and additional training in the use of these systems.
As the data has consistently shown, antiquated invoicing processes create an incredible administrative burden for clinical research sites. Year after year, sites have also emphasized the need for more frequent payments in order to support their ability to recruit patients, collect superior data, and achieve overall high-quality study performance. They are seeking payment solutions that enable streamlined and efficient payment processes, reducing administrative burden and allowing them to focus on the true driver of clinical research: the patient.
Manual invoicing processes create an incredible burden for clinical research sites. In 2016, survey data showed that 50 percent of site respondents created invoices manually. Survey data from 2017 showed some improvement, with 43 percent of respondents indicating that they were creating invoices manually. Four out of five site respondents shared that reducing manual invoicing processes and transitioning toward automated invoicing would be extremely or very beneficial. In addition, 90 percent of sites shared that they want more frequent payment terms. When the need for improvement is this great, change must be made. Though improvement is occurring, with more sponsors and CROs implementing automated invoicing processes, the change is slow to come and only small improvements are being realized by sites each year. When the majority of sites have clearly and consistently identified their need for more timely payment terms in order to support their ability to recruit patients, collect superior data, and achieve overall high-quality study performance, faster change is urgent.
Sites that must engage in manual invoicing processes have less time to dedicate to recruiting and treating patients. Data from the 2017 survey shows that 74 percent of sites spend at least 1 hour per month compiling and creating invoices for each study they conduct. These numbers become even more staggering when we look at responses from sites outside of the United States: one in four OUS sites spend 7 hours or more per month creating invoices for each study. This time expenditure adds up quickly and has a direct impact on sites’ ability to conduct quality research. The more time sites – within or outside of the U.S. — are required to spend engaging in unnecessary manual processes, the fewer resources they will have to achieve sponsors’ study deliverables. Despite the common requirement that OUS sites must invoice for all services completed, thus creating an even more burdensome manual invoicing process, only one out of 102 total OUS respondents said they prefer to be paid less frequently.
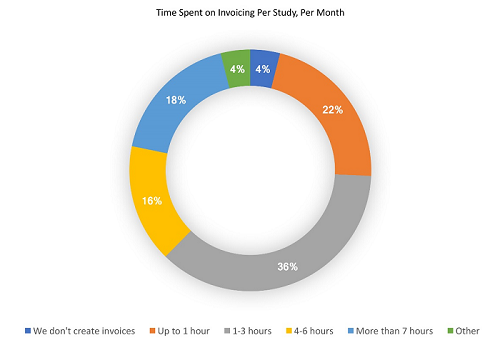
Insufficient payment terms have also played a role in creating the one-and-done site problem, where a research site or investigator conducts only one clinical trial before ceasing operations. It is impossible to ignore the need for improvement when nearly eight out of 10 sites shared that they request a monthly payment schedule during budget negotiations every time or most of the time, and nine out of 10 sites want to work with partners who pay more frequently.
Pain points throughout the invoicing and payments process create end-to-end delays, making it nearly impossible to achieve timely payments to sites. Further, site resources are diverted away from treating patients. Sponsors and CROs that seek out and utilize solutions to these problems will emerge as preferred business partners and contribute to site sustainability and success. By addressing the pain points sites have indicated as critical to their success, these organizations align themselves as sponsors or CROs of choice and will draw the attention of highly qualified sites to conduct studies.
It is also paramount that sponsors, CROs, solution providers, and sites alike address, acknowledge, and seek solutions to the inevitable time required for sites to learn how to utilize and implement new technology. Whenever possible, this time should be incorporated into budget negotiations and reflected in site payments. Just as every employee receives on-the-job training for a new position, the necessary site personnel must receive training to ensure they are prepared to use the solutions provided to begin automating invoice processes and ensure they will be successfully implemented.
There has been progress in these ever-important areas that drive site success and sustainability. However, more work is needed. The data indicates the need for a renewed directive for automated invoicing options and payments made to sites within 30 days. SCRS will continue to advocate on behalf of these key improvements that will help to realize a sustainable and barrier-free process for all players — most importantly, the patients we treat.
To access the SCRS-Greenphire white paper, Financial Barriers to Site Sustainability, Patient Experience and Overall Trial Success, click here.
About The Author:
 Jessica Knott is senior communications manager at the Society for Clinical Research Sites (SCRS). With a background in professional writing and marketing, she began her career in clinical research at Johns Hopkins University (JHU), managing research studies under a large, federally funded task order. She then moved on to a position at the JHU School of Medicine, writing and negotiating subawards. In 2017, Knott joined SCRS as senior communications manager, overseeing all company publications and social media efforts. In addition, she cofounded a small wellness organization in 2014 and manages all company communications. She regularly works with businesses to craft their written content, writing articles, website copy, and standard operating procedures.
Jessica Knott is senior communications manager at the Society for Clinical Research Sites (SCRS). With a background in professional writing and marketing, she began her career in clinical research at Johns Hopkins University (JHU), managing research studies under a large, federally funded task order. She then moved on to a position at the JHU School of Medicine, writing and negotiating subawards. In 2017, Knott joined SCRS as senior communications manager, overseeing all company publications and social media efforts. In addition, she cofounded a small wellness organization in 2014 and manages all company communications. She regularly works with businesses to craft their written content, writing articles, website copy, and standard operating procedures.
