What Janssen Learned From Listening To Patients In Pre-Approval Access
By Beverly Harrison, Janssen Pharmaceuticals

Patient-centricity is a much-used buzz phrase these days; however, the truth is that often the patient voice is neither heard nor heeded when creating initiatives to help them. This can be particularly true in the pre-approval access space, where seriously ill patients who have exhausted all options are seeking access to potentially lifesaving medicines. In 2018, a first of its kind meeting was convened to hear the global voice of patients in pre-approval access (PAA). In the EU, PAA is often known as compassionate use (CU), expanded access (EA), or managed access (MA), where the regulatory landscape is widely diverse in its requirements for seeking this form of access.
With support from senior leadership in our organization, the Janssen Pharmaceuticals Companies of Johnson & Johnson, Office of the Chief Medical Officer, along with the Division of Medical Ethics at NYU School of Medicine and patient advocates, partnered with one another to conduct the EU PAA Summit in November 2018 in Brussels.
As we learned from the pioneering HIV advocates in the 1990s, “nothing about us without us.”1 How can we really know what is critically important to patients facing such potentially life or death situations unless we ask them directly? As Francois Houÿez, Information & Access to Therapies Director & Health Policy Advisor, European Organisation for Rare Disease (EURORDIS), has commented, “That’s the worst case: you are dying of a disease, you know that there is a drug out there that could help you but it is not yet available….”.
In the spirit of our goal of sharing our learnings, I provide below a summary of our preparations, learnings, and what we can do together to continue advancing our listening.
Understanding Patient Perspectives
In collaboration with Janssen Patient Advocacy and Engagement colleagues in Europe, we identified patients/patient groups interested in CU and assessed the landscape from the patient perspective. The EURORDIS CU position paper from April 2017 titled Early access to medicines in Europe: Compassionate use to become a reality was very useful in understanding the landscape. We learned there are great disparities in terms of timely access to new medicines in Europe and that there has been little progress in PAA observed over the last 10 years.
With this front of mind, a Patient Steering Committee was formed to co-create the agenda for this Patient Summit, with the goals of advancing the global dialogue on PAA, sharing best practices, and inspiring real action.
Key Findings
Throughout the course of a one-day meeting with more than 50 patient advocates, we discussed the key topics noted below.
- What is Pre-Approval Access?
- Current Landscape of Pre-approval Access
- Innovative Partnerships, Pilots, and Programs
- How to Affect Change
Unsurprisingly, patient advocates do indeed inspire action. Sessions with EURORDIS, European Patients Forum (EPF), The Patvocates Network, and other leading European organizations identified opportunities for collaboration on behalf of patients seeking PAA.

The most impactful session of the meeting was on “Dialogue to Effect Change,” which involved patient advocates, academia, and industry. The key themes from this rich discussion are summarized below and serve as a call to action for all stakeholders.
- Patients made it clear that it is time for regulators and pharmaceutical companies to take action on a policy level with the European Medicines Agency (EMA) and member states and to involve patients in early stages of development.
- New collaboration models involving patients and patient advocacy groups (PAGs) are needed. PAGs are the missing link to connect all stakeholders and have a very important role to play in affecting change. PAGs challenged themselves to leverage the lessons learned from the HIV activists and become agents of change working with key stakeholders.
While many of the recommendations from the Patient Summit will require a multistakeholder approach and established frameworks for working together, such as the Innovative Medicines Initiative (IMI), Janssen did take away the specific action items below for our organization, which we continue to advance and explore.
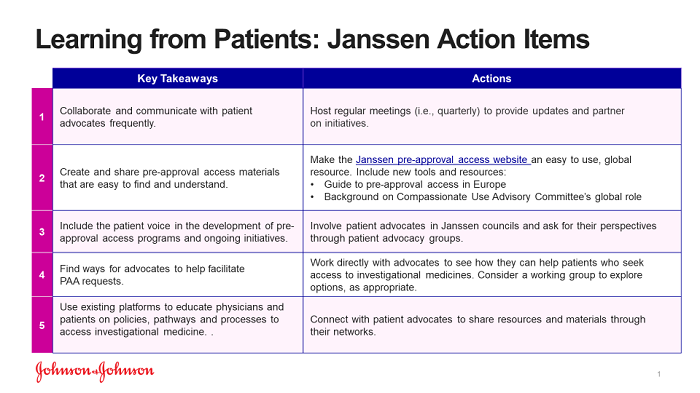
A Bold Call To Action On Behalf Of Patients
Let’s come together now with the sense of urgency needed by patients to find innovative ways to address the challenges in the PAA space. We suggest exploring public-private partnerships to leverage existing collaboration models, including, but not limited to, IMI, the European Federation of Pharmaceutical Industries and Associations (EFPIA), and the European Patients Academy on Therapeutic Innovation (EUPATI). Collaboration with such organizations may advance our ability to address the PAA education and resource challenges and to develop a path forward in this space. Leveraging established frameworks allows participation from patients and patient advocates, developers, academia, health authorities (HAs), and health technology assessment (HTA) bodies.
Together, we need to ensure that patient voices are heard at every step to realize equitable access to information, consistent mechanisms for fair review, global alignment, and harmonization. The time to act is now; please join us. As Dr. Paul Janssen noted, “patients are waiting” and they cannot and should not wait another 10 years for progress in the PAA space.
References:
- Lessem, E. (2019, May). Nothing About Us WIthout Us. tagline, p. 2.
For More Information:
About The Author:
 Beverly L. Harrison is head of the Patient Support Group in the Office of the Chief Medical Officer at Janssen R&D, the research arm of Johnson & Johnson. She reports directly to the chief medical officer and focuses on developing strategies to understand and support the needs of patients by working with internal and external stakeholders, including a special focus on engaging patient advocacy organizations. Harrison is a research, development, and nonprofit patient advocacy professional with over 25 years of leadership experience integrating team efforts across functions with increasingly high levels of responsibility for impacting the policies and performance of pharmaceutical, CRO, and nonprofit organizations.
Beverly L. Harrison is head of the Patient Support Group in the Office of the Chief Medical Officer at Janssen R&D, the research arm of Johnson & Johnson. She reports directly to the chief medical officer and focuses on developing strategies to understand and support the needs of patients by working with internal and external stakeholders, including a special focus on engaging patient advocacy organizations. Harrison is a research, development, and nonprofit patient advocacy professional with over 25 years of leadership experience integrating team efforts across functions with increasingly high levels of responsibility for impacting the policies and performance of pharmaceutical, CRO, and nonprofit organizations.
