What Skills Are Needed To Effectively Manage An eTMF?
 A recent Internet search for job postings using the term “trial master file” (TMF) yielded several opportunities in my geographic region with the following range of titles:
A recent Internet search for job postings using the term “trial master file” (TMF) yielded several opportunities in my geographic region with the following range of titles:
- TMF associate
- TMF coordinator
- TMF manager
- TMF specialist
- clinical project associate
- associate project coordinator
- clinical project coordinator
- Manager, TMF Operations
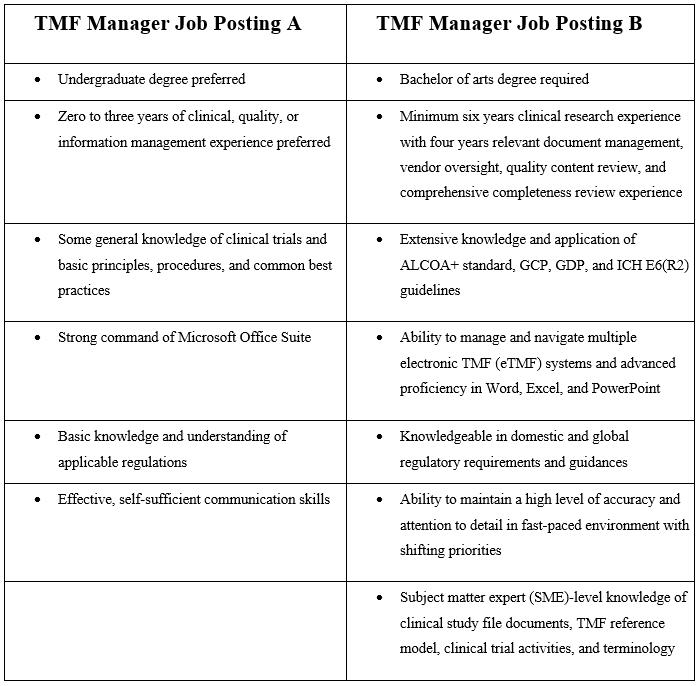
The requirements for each job posting varied greatly. In fact, the requirements for two different postings with the same exact job title — “TMF manager” — differed considerably:
Finally, one job description included:
- support eTMF systems, TMF inspection readiness, and the head of TMF operations in day-to-day activities
- manage TMF projects, assist with overall change management, and build collaborative relationships with cross-functional teams and external vendors
- support TMF operations during eTMF implementation, audits, and/or regulatory inspections
- ensure functional compliance with company TMF SOPs, KPIs, relevant TMF process-supporting guidances, and applicable global regulations
- perform risk-based quality content reviews and monitor internal/external KPIs
- ensure the company TMF is inspection-ready at all times
- manage TMF room entry logs, document check-in/check-out logs, access, and temperature logs
- represent TMF operations at study team meetings, participate in collaborative efforts, and play an important cross-functional role in TMF document retrieval and management
- ensure documents which fail TMF quality content and/or TMF inspection-readiness review are effectively remediated by internal/external representatives
- monitor and identify study-specific TMF trends and escalate concerns to head of TMF operations
- coordinate the long-term storage archival of original documents and maintain document integrity, per mandatory retention policies
- participate in TMF educational workshops and trainings.
Which TMF manager description from the chart above — A or B — would be the appropriate choice for this job? The answer lies in one of the basic tenets of GCP: E6(R2) Section 2.8: “Each individual involved in conducting a trial should be qualified by education, training, and experience to perform his or her respective task(s).”1
The Internet search above revealed to me what I suspected: there are no formal definitions around the education, training, and experience for TMF owners. It also confirmed impressions accumulated in my career: first, this job requires an incredibly wide range of skills and experiences; second, this job requires at least basic understanding of each and every functional area that can touch a clinical trial, from discovery and CMC (chemistry, manufacturing, and controls) to commercialization and asset management; and third, unless you plan proactively to have the right TMF, the right resources, and the right organizational attitude toward the TMF, it becomes a task that is often seen as a burden.
The Missing Link
In line with that logic, I believe one critical SME has not been included proactively to date when adopting an eTMF, whether outsourced to your CRO to manage, to a third-party vendor, or maintained and managed in house at the sponsor (or some combination thereof — which is an article for another day!). Who is that SME? Take a guess from the options below:
- Clinical Trial Management
- QA
- IT
Both 1 and 2 serve critical roles, but I find IT is an afterthought at best, and a completely missing link at worst. Why do I think that? Because clinical operations personnel are SMEs in their universe but not in the realm of all things IT-related. Further, IT professionals do not often “speak clinical.” So they need clinical operations partners who can explain the structure, purpose, attributes, and metadata an eTMF can provide. For example, how many countries will we enroll in? What is the trigger for updating the master informed consent form? How often and how must we run QCs of our eTMFs?
This shift in resourcing requires buy-in from senior IT leadership within the organization and an understanding of the scope, skills, and resourcing required of the person(s) who will support the installation, integration, validation, and training of the eTMF, not to mention the likely system changes/upgrades an eTMF requires during a clinical trial.
According to a recent article by Oliver Pearce at Montrium, a summary of the business rationale and justifications for moving (from paper-based trial master files) to eTMF systems can include the following:2
- Reduced business risk – Systems provide confidence that you have met the regulatory compliance requirements.
- Enhanced artifact quality – Automated systems have been proven to make fewer errors than manual paper handling processes and have the ability to implement automated quality control processes.
- Improved team productivity – Sharing and viewing documents anytime, anywhere, from any device is faster than manual paper retrieval.
- Reduced auditing and reporting costs – Automated reporting and electronic retrieval of eTMF content can significantly reduce auditing and reporting labor and travel costs.
Further, in a 2016 CenterWatch white paper entitled “eTMF Adoption and Integration Accelerating,” 3 the authors noted there are keys to successful implementation, such as including SMEs from each functional area to contribute to the new business process development and system implementation. “Change management must be fully planned; eTMF adoption takes a village,” one sponsor company was quoted as saying, as well as “identifying key stakeholders in the business across many functions takes time, and then engaging them during the life cycle of the implementation phase is important.”
Once you have the right people owning and maintaining the TMF, don’t dictate quality review, deadlines, and tasks, and don’t let the focus change every time the winds shift around the perceptions of documentation regulations and best practices. Train, resource, and empower your TMF owners — then let them do their jobs, and address their needs as they arise during the clinical trial. They are, in fact, the SMEs of the TMF — if they are qualified by education, training, and experience to do so.
Bottom line: proactively understand, prioritize, resource, and champion your TMF team. When audit or inspection time arrives, you will be glad you did.
References:
- www.fda.gov/scienceresearch/specialtopics/runningclinicaltrials/guidancesinformationsheetsandnotices/ucm219488.htm
- https://blog.montrium.com/blog/what-is-etmf-software-and-does-my-organization-need-to-implement-one
- Originally published in the December 2016 issue of The CenterWatch Monthly
About The Author:
 Audrey Rossow is the owner of A Rossow Consulting, LLC, located in central Massachusetts. She has more than 25 years of experience in pharmaceutical and biotech clinical development, from Phases 1 to 3b. Her core work is in project management and clinical operations. She is passionate about site engagement and support, patient recruitment and retention, and sponsor oversight of their CROs. She can be reached at audrey@arossowconsulting.com, and her website is www.arossowconsulting.com.
Audrey Rossow is the owner of A Rossow Consulting, LLC, located in central Massachusetts. She has more than 25 years of experience in pharmaceutical and biotech clinical development, from Phases 1 to 3b. Her core work is in project management and clinical operations. She is passionate about site engagement and support, patient recruitment and retention, and sponsor oversight of their CROs. She can be reached at audrey@arossowconsulting.com, and her website is www.arossowconsulting.com.
