Why Every Pharma Needs A Service Management Group (SMG) For Successful Outsourcing
By Krishnan Rajagopalan, Ph.D., president, Life Sciences – Sourcing Advisory and Consultancy Services

The pharmaceutical industry is in the throes of innovation and transformation. Targeted therapeutic approaches for cancer, rare diseases, and rapidly evolving genomics and molecular medicine are translating into precision treatments and individualized therapeutic protocols. Gene therapy and gene editing are beginning to transform the industry at a fundamental level. Decentralized clinical trials (DCTs) are aiding the transformation, facilitating a patient-centric approach, and leveraging a variety of digital technologies.1
Over the past decade, large pharma companies’ share of the industry’s clinical pipeline has declined, with the share of pipeline for the top 15 pharma companies shrinking from around 40 percent to around 20 percent from 2011 to 2021.2 Looking ahead, biotech has a significant proportion of advanced molecules in development across areas including cell and gene therapy, monoclonal antibodies, and DNA and RNA therapeutics. While large pharma companies are expected to grow R&D spending at a rate of 4 percent annually — to $234 billion for the industry in 2025 — R&D spending in biotech that typically falls under small and medium enterprises (SMEs) is forecast to grow twice as fast, at up to 8 percent per year.3
Industry leaders across small, medium, and large pharma companies are focusing on targeted objectives and tangible outcomes across R&D functions. One of the key levers for realizing their objectives is to leverage and align outsourced partners with their own strategic objectives and outcomes. There is increasing expectation of companies to use their partners for not only providing transactional operational services and outcomes but also to participate in and drive some of their transformation programs (e.g., automation, DCTs, risk- based monitoring, and hybrid/virtual operating models).
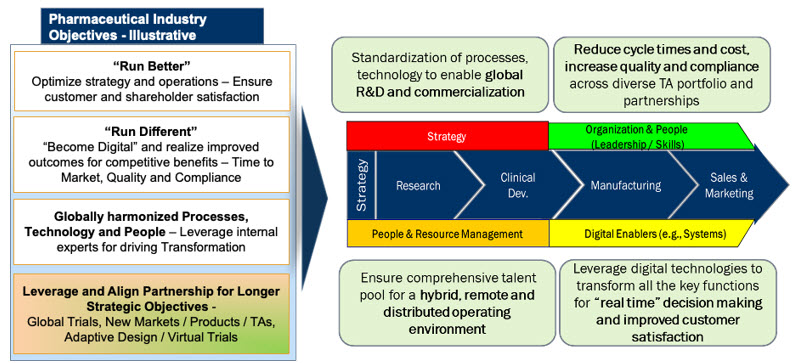
As a significant portion of R&D work is outsourced to external partners such as CROs and functional service providers (FSPs), it is imperative that life science companies have a dependable set of global and regional external partnerships that are flexible and scalable.2 These partnerships need to fit the diverse needs of small, medium, and large companies and a variety of “future-ready” operating models.
How A Service Management Group (SMG) Can Solve Common Outsourcing Issues
A comprehensive end-to-end sourcing and outsourcing approach that is strategic in all its elements can allow life science companies to leverage best practices and realize their clinical transformation objectives. While a great deal of focus is placed on the request for proposal (RFP) process, pricing, and contract negotiations, one of the critical areas not getting enough attention is the design and development of an internal, dedicated Service Management Group (SMG) that is adequately staffed, trained, and empowered to manage the multiple suppliers and the strategic objectives of the outsourcing programs. The size and shape of the SMG depends on the size of the company or the functions that are being serviced by the group, the number of outsourced suppliers, and the strategic nature of the relationships.
The lack of an optimally designed and performing SMG leads to multiple challenges in managing the various supplier partnerships, some of which are outlined below:
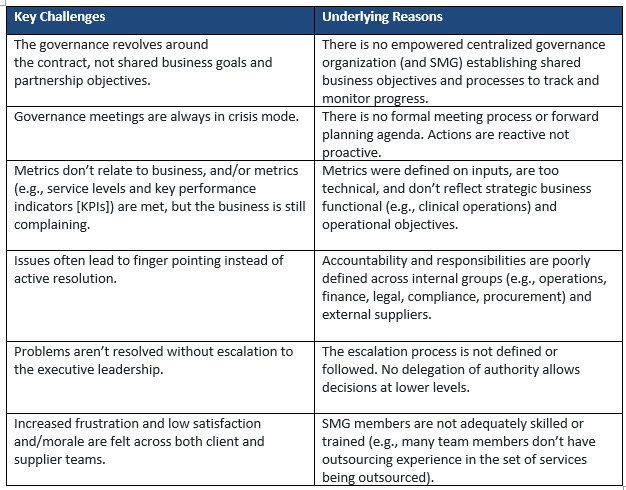
When no SMG is established, implementations get stalled, client stakeholders and supplier teams are frustrated, and the entire schedule for achieving expected business benefits is delayed. This could lead to internal turnover of staff, low morale, and an outsourcing program that is not delivering the business outcomes.
Putting An SMG Into Action
Effective sourcing and supplier management is critical to ensure R&D and other functions can achieve their strategic business and sourcing/outsourcing objectives. SMGs can play a pivotal role in enabling and ensuring realization of objectives.
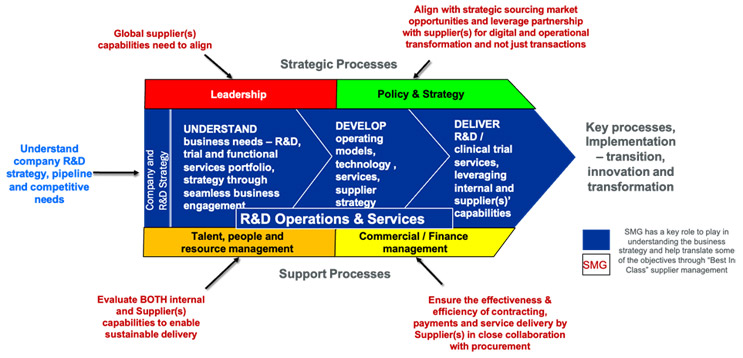
The key objectives of an SMG are:
- Control the work that is initiated with the service providers.
- Ensure the service providers deliver to business requirements as contracted.
- Optimize the delivery of the contract, such that value is driven from the contracts.
- Control the budget spend with the providers.
- Drive continuous improvement and innovation from the providers.
- Ensure the business and the organization have a uniform approach to the providers.
- Maintain central control to negotiate additional services from the providers, if required.
- Resolve issues in a consistent, effective manner.
- Monitor performance of the providers.
Some early advantages of having an SMG group include direct interaction with business stakeholders early on, participation in the sourcing process, a deep understanding of the service providers and their capabilities and drawbacks, and familiarity with the contracts they will be managing in the future.
The main functions of an SMG are:
- Business Relationship Management: The SMG is the face of the business functions and users, providing advice and translating the business needs into sourcing/outsourcing requirements.
- Strategy and Process Controls: The SMG ensures that the oversight controls, standards, compliance requirements, and transformation objectives are aligned between the client and provider(s).
- Contract Management: The SMG ensures that the organization, service providers, and third-party providers/subcontractors execute their contracts in full and that effective controls exist to manage the contract risks and provide proactive mitigations.
- Financial Planning: The SMG ensures financial procedures and controls are observed, invoices are paid, and the contract(s) are optimized continuously.
- Program(s) Control: The SMG ensures plans for operating model changes (e.g., digital clinical trials and risk-based monitoring) are integrated with the service providers’ processes and ensures a coordinated view of operational project and near real-time tracking of metrics and outcomes are maintained.
- Service Delivery: The SMG is also the face of the organization to providers, managing day-to-day services and the translation of business requirements into services from suppliers.
- Creation And Maintenance Of Seamless Interfaces With Internal Groups: The SMG ensures seamless interactions among stakeholders (e.g., legal, procurement, compliance, and functional groups like data management and quality). The early design of the SMG should involve clear delineation of roles and responsibilities of the various stakeholder groups/functions to the level of individual roles through a RACI (Responsible, Accountable, Consulted, and Informed) chart. The RACI is typically developed in collaboration with all the key stakeholder groups, leveraging best practices in organizational change and communications management. In our experience, strategic outsourcing programs are successful when the business’ functional objectives and outsourcing objectives are aligned through specific metrics for both the functional group doing the outsourcing and individual performance objectives of the team members (e.g., SMG).
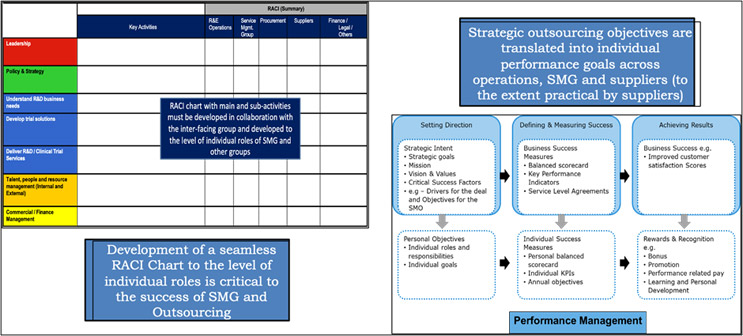
- Effective Governance With All Providers: A key function of the SMG that sometimes gets less attention is the design and implementation of a robust joint governance structure with active participation from senior leadership and operational leaders from both the pharmaceutical company and the provider organizations. We have seen that the presence of structured joint governance forums, deliverables, and RACI typically leads to sustained benefits from the strategic sourcing and outsourcing programs and the overall business objectives.
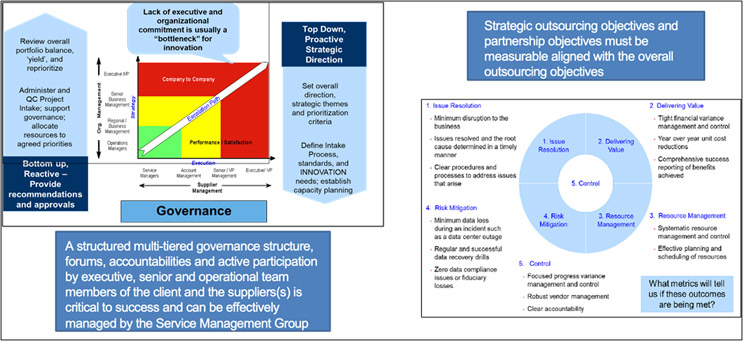
Finally, client organizations and major functions that are trying to outsource many of their activities and deliverables (e.g., R&D, commercial, IT, legal, and human resources) can benefit from the development of a comprehensive balanced score card (BSC), which documents and shares objectives with tangible and measurable metrics.

We are at an exciting stage of transformation across the pharmaceutical value chain with tangible options for leaders to improve their individual performance and the company’s overall competitive position. Given that many of the business functions across the value chain are leveraging outsourcing as a strategic lever to realize their objectives, it is important that they design and implement an empowered SMG to manage and nurture all their external alliances and service providers. An optimally designed and strategically driven sourcing and outsourcing program along with an effective SMG will ensure that the industry can continue to deliver safer and highly effective medicines to their patients — faster and at an affordable cost.
About the Author:
 Krishnan Rajagopalan, Ph.D., is the president of Life Sciences – Sourcing Advisory and Consultancy Services and brings more than 27 years in management consulting, strategic sourcing and outsourcing advisory (BPO, ITO, CRO, FSP) and global services delivery to Fortune 100 clients. He has advised small, medium, and large life sciences clients on strategy, organizational change and communications, operational model design and implementation, and strategic sourcing and outsourcing services. Krishnan also delivered full-service CRO, CDM, pharmacovigilance and/safety, biostatistics and /programming, MW, regulatory affairs, commercial services, and IT/digital services to more than 100+ life sciences companies for Fortune 500 global services providers from India, China, Argentina, Europe, and the U.S. He can be reached via email, over mobile at +1 908 380 3343, and on LinkedIn.
Krishnan Rajagopalan, Ph.D., is the president of Life Sciences – Sourcing Advisory and Consultancy Services and brings more than 27 years in management consulting, strategic sourcing and outsourcing advisory (BPO, ITO, CRO, FSP) and global services delivery to Fortune 100 clients. He has advised small, medium, and large life sciences clients on strategy, organizational change and communications, operational model design and implementation, and strategic sourcing and outsourcing services. Krishnan also delivered full-service CRO, CDM, pharmacovigilance and/safety, biostatistics and /programming, MW, regulatory affairs, commercial services, and IT/digital services to more than 100+ life sciences companies for Fortune 500 global services providers from India, China, Argentina, Europe, and the U.S. He can be reached via email, over mobile at +1 908 380 3343, and on LinkedIn.
References:
- Best Practices for Sourcing & Outsourcing In Clinical Operations – Clinical Leader, Oct 2021
- CROs and biotech companies: Fine-tuning the partnership – McKinsey and Company, Article June 9, 2022.
- Evaluate Pharma data base, Evaluate May 2021
- Best Practices in utilizing the FSP model: Clinical Operations, Pharmacovigilance, Regulatory. and other areas – Applied Clinical Trials, November 16, 202
