Your Guide To Harnessing Clinical Trial Sensor Data Better
By Jennifer Goldsack, Digital Medicine Society

Technological advances in wearables and sensors are speeding the development of digital measures and endpoints throughout the drug development process and across therapeutic areas and patient populations. The pandemic subsequently drove broader acceptance from patients, researchers, clinicians, regulators, and the general public about the role of this technology and the prevalence of decentralized clinical trials.
In October 2019, the Digital Medicine Society (DiMe) launched our crowdsourced library of digital endpoints to shine a light on digital endpoints being used in industry-sponsored trials of new medical products and galvanize the field around specific measures to speed adoption.
Since then, the endpoints library has tracked a tenfold growth in the use of digital endpoints by industry, moving from 34 in October 2019 to 325 at the last update in May of this year. Simultaneously, the number of sponsor organizations relying on digital endpoints to deliver better information, sooner, about how well their medical products are performing has risen from 12 to 96.
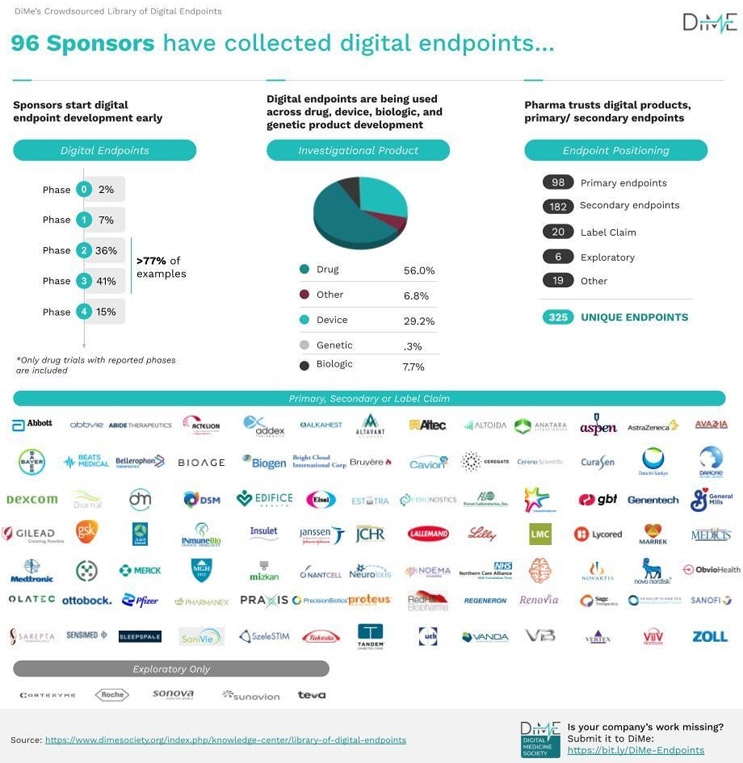
The embrace of digital endpoints from medical product developers ranging from pharma companies to manufacturers of gene therapies should be no surprise. Digital endpoints offer substantial opportunities to improve what we know about patients’ biologic processes and responses, as well as how they feel, function, and survive in both clinical trials and routine clinical care. This opens the door for better go/no-go decisions, increasing the possibility of technical success as well as the identification of digital phenotypes that can improve future drug discovery in certain well-defined populations.
Beyond the clinical benefits, digital endpoints offer opportunities to improve access to clinical trials, driving more generalizable findings and speeding recruitment timelines. Higher-resolution flows of data offer the possibility of reducing sample sizes, speeding timelines further while slashing costs. And beyond the promise of reducing the time it takes for patients to access new drugs that address their symptoms, digital endpoints also support decentralized approaches to clinical trials, which are essential in the wake of the COVID-19 pandemic.
Achieving A Tailored Approach To Successfully Leverage Sensor Data
But with the rapid embrace of digital endpoints by industry, the FDA, and the European Medicines Agency (EMA), we have to ask whether the life sciences industry has the necessary infrastructure in place to leverage the volume and power of sensor-generated data to drive better decisions, faster, in medical product development.
Currently, while the majority of this sensor data is “in the cloud,” it is often in native formats unique to each sensor technology and in proprietary clouds owned by the manufacturer. Across both healthcare and clinical research, these data can only be accessed by bespoke point solutions – often provided by an intermediary solution provider – and while this cumbersome approach works today, it will not efficiently or effectively scale as our industry’s reliance on sensor-generated data and digital endpoints grows.
To address this challenge, the Digital Medicine Society (DiMe) led a group of key stakeholders from across the health-tech continuum – Amazon Web Services, Elevance, Evidation, FDA, Moffitt Cancer Center, Human First, IEEE Standards Association, Medable, Oracle, Open mHealth, Takeda, Savvy, and the U.S. Department of Veterans Affairs (VA) – to get ahead of the impending threat of the scale of sensor-generated data in healthcare and research. They surveyed use cases across the care continuum and released a comprehensive set of four open-access toolkits to support the successful scaling of sensor data to drive better decisions, faster, in patient care and clinical research.
Introducing The Sensor Data Integrations Toolkits
The DiMe team took a comprehensive look at this topic and developed the Sensor Data Integrations Toolkits, which are four comprehensive toolkits to guide data producers, processors, and consumers to use the influx of data from the increased use of wearables and digital sensing products at scale. The tool most relevant to those working on clinical trials is the Data Flow Design tool.
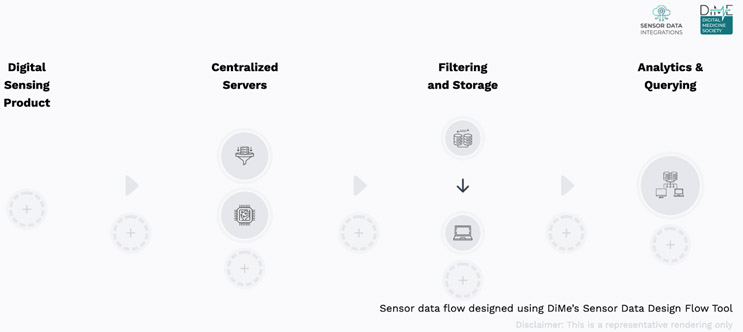
This tool helps you map the flow of sensor data from any connected sensor technology you choose through to a final data set for analytics and querying, whether within a care delivery or research setting. First, you answer a few questions to build out the right steps, such as: Does your digital sensing product require an app or hub to transmit data from the measurement tool to you? And does data generated by your digital sensing product flow through servers belonging to the manufacturer of the measurement tool? Then, you have the option to use a user-friendly, interactive design tool to build and annotate your bespoke sensor data flow – from product to servers to filtering and storage to analytics and quarrying – for documentation and collaboration with your team and partners. This helps you identify a process to make working with digital health partners seamless.
The Sensor Data Integrations Toolkits are comprehensive and will help realize the promise of sensor-generated data to drive better decisions, faster, to improve healthcare delivery and research. Beyond the Data Flow Design tool, the Sensor Data Architecture Toolkit offers a Logical Data Architecture tool, which is an ecosystem-level strategy for deriving value from the data you produce irrespective of platform, operating system, file structure, or database technology, and a Reference Data Architecture tool to share examples of ways that the types of sensor data that you generate have been successfully integrated into the health data ecosystem to power clinical decision-making.
The Sensor Data Integrations Implementation Toolkit, includes an overview of the accessible, relevant, and trustworthy (ART) criteria that are critical to delivering data effectively to downstream partners. It also includes short “cheat sheets” of key considerations and best practices aligned with each criterion, an ART Criteria Prioritization tool, and real-world examples so you can see the ART criteria in action.
The Sensor Data Standards Toolkit offers an interactive landscape of standards, which are all of the standards relevant to sensor data integrations in an interactive landscape tool. DiMe also developed an interactive library of standards and is committed to keeping this up to date as new standards are developed.
The last toolkit, Organizational Readiness for Sensor Data Integrations, offers a capabilities maturity model to guide understanding of your partners in the downstream market and a capabilities maturity calculator, which is an assessment tool to benchmark your partners’ preparedness and guide you to the right resources to meet them where they are for shared success.
Digital data and technologies provide real opportunity for change and a move toward optimizing human health. However, this promise will not be realized organically and indeed may cause more inefficiency — or worse, harm — if not well-deployed. The digital medicine community has the expertise to solve these problems, but the coordination isn’t there. DiMe was created to harness the power of the diverse stakeholders that comprise the digital medicine community, evaluate and prioritize big problems that digital medicine is positioned to solve, and bring together key stakeholders across the spectrum to create tools, resources, educational material, and case studies to guide the industry at this critical point in time.
The tools outlined above are an easy jumping off point for organizations to evaluate their landscape and put plans in place to charge forward. But harnessing sensor data is just one example of the way that DiMe is creating solutions to problems before they impede the potential of the digitization of healthcare to improve lives.
About The Author:
 Jennifer C. Goldsack, MChem, MA, MBA, OLY, co-founded and serves as the CEO of the Digital Medicine Society (DiMe), a 501(c)(3) non-profit organization dedicated to advancing digital medicine to optimize human health. Her research focuses on applied approaches to the safe, effective, and equitable use of digital technologies to improve health, healthcare, and health research. She is a member of the Roundtable on Genomics and Precision Health at the National Academies of Science, Engineering and Medicine and serves on the World Economic Forum Global Futures Council on mental health.
Jennifer C. Goldsack, MChem, MA, MBA, OLY, co-founded and serves as the CEO of the Digital Medicine Society (DiMe), a 501(c)(3) non-profit organization dedicated to advancing digital medicine to optimize human health. Her research focuses on applied approaches to the safe, effective, and equitable use of digital technologies to improve health, healthcare, and health research. She is a member of the Roundtable on Genomics and Precision Health at the National Academies of Science, Engineering and Medicine and serves on the World Economic Forum Global Futures Council on mental health.
