ABOUT ROCHE
Throughout our 125-year history, Roche has grown into one of the world’s largest biotech companies, as well as a leading provider of in-vitro diagnostics and a global supplier of transformative innovative solutions across major disease areas. Our commitment to our people, partners, stakeholders and, most importantly, our patients remains as strong as it was on the first day of our journey.
Accelerating access to innovation with the patient in mind
navify ® Digital Solutions from Roche Diagnostics are designed to transform laboratory and clinical data into actionable insights, delivering operational and financial value, and helping to personalize care along each patient’s healthcare journey.
The Roche Digital Biomarker Solution for clinical trial sponsors provides promising reliability and early clinical validity in remotely measuring disease progression for individuals with early Parkinson’s disease. This innovative approach can potentially expedite clinical research and provide greater confidence in measuring effect size.
With a robust track record in clinical trials, our solution has amassed over 14,000 months of data and has been prominently featured in more than 50 scientific publications, posters, and presentations since 2015.* We are committed to facilitating research by providing researchers and clinical trial sponsors access to the Roche Digital Biomarker Solution, empowering them to accelerate their groundbreaking studies.
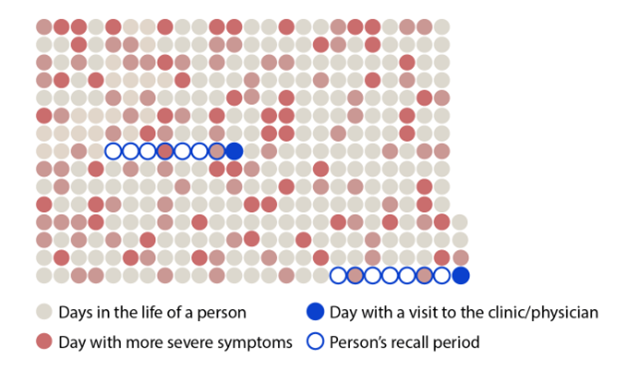
365 Days In The Life Of A Patient
Individuals grappling with neurological conditions like Parkinson's disease, may struggle to accurately remember and report the manifestation and severity of their symptoms during periodic in-clinic visits. The reliability of occasional in-clinic assessments spanning months is prone to inconsistencies between raters and within the same rater over time.
The remote at-home digital assessment solution by Roche offers advantages for life science and clinical trial sponsors. This approach may provide clinical trial researchers with more meaningful insights and increased sensitivity.1-2 The Roche Digital Biomarker Assessment Suite and Service is a comprehensive disease progression measurement solution for movement disorders like Parkinson's disease. The suite includes active in-clinic tests supervised by professionals, as well as active and passive monitoring integrated into subjects' daily routines at home.
This holistic solution, equipped with a digital health tool and clinical trial services, is thoughtfully designed to support patient and site workflows throughout clinical trials, encompassing technology, data collection, and analytics.
Transform your clinical trials with a remote at-home digital assessment solution and start unlocking data collection for more meaningful insights and heightened sensitivity.
References:
- Dorsey ER, Venuto C, Venkataraman V, Harris DA, Kieburtz K. Novel methods and technologies for 21st-century clinical trials: a review. JAMA Neurol. 2015 May;72(5):582–8.
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society UPDRS revision task force. movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008 Nov 15;23(15):2129–70.
Disclaimer
The Roche Digital Biomarker Solution for Parkinson’s disease is intended to collect, store and process digital clinical data to support exploratory research in clinical trials. This solution is not meant to be used for diagnosis and treatment decision making for individual patients.
CONTACT INFORMATION
Roche Information Solutions
2801 Scott Blvd
Santa Clara, CA 95050, CA 95050
UNITED STATES
Phone: (408) 643-5742
Contact: Sandy Tan