Human AME TadioLabeled Studies
PRODUCTS
-
Anritsu's aerosol checkweigher is engineered for accurate weighing of aerosol cans in pharmaceutical applications. It features a star wheel mechanism that feeds cans onto the weigh table at a constant speed and with uniform spacing. This design, combined with a high precision electromagnetic weigh cell, ensures a throughput of up to 150 cans per minute, with a weighing accuracy of ± 10 mg. The checkweigher has a compact footprint, integrating within the same frame a reject mechanism and confirmation function, ensuring that only correctly weighed products pass through. Underweight and overweight cans are automatically directed into two separate bins located beneath the weigh table. The system is compliant with federal regulation 21 CFR Part 11 for the integrity of electronic records and signatures, with features such as password authentication, audit trail of operation data, and encryption/decryption of exported data.
-
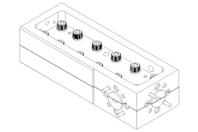
The C10-1000WG is a WR10 10dB waveguide coupler featuring high directivity over a 75-100GHz operational bandwidth built with high precision and then gold plated for high corrosion resistance.
-
Review how a comprehensive cGMP manufacturing facility is poised to support the development and manufacture of your mammalian cell program.
-
Learn how to achieve unmatched speed and precision with PG&O's new double-sided polishing technology. Learn more about faster production and superior quality for your optics.
-
The RFS-2G42G5050X+ by Mini-Circuits is a next-generation solid-state high-power signal source designed for industrial, scientific, and medical applications operating within the 2400–2500 MHz ISM band.
WHITE PAPERS AND CASE STUDIES
-
TOC Reduction For Solar Photovoltaic Plant
Atlantium Technologies installed UV systems in an Indian solar photovoltaic manufacturing plant to remove impurities from ultrapure water, effectively reducing TOC levels and providing high microbial reduction. Their HOD UV technology has been proven effective in water treatment.
-
Clinical Phase Manufacturing With A Self-Contained Cleanroom Facility
Explore the development of a mobile Biosafety Level 2+ (BSL-2+) facility adhering to cGMP for early-phase clinical trial manufacturing.
-
Implementation Of An X-Ray Inspection System For Mixed Nut Products
Learn about an inspection system that addressed the key concern of an Italian nut supplier of ensuring no foreign contaminants were present in the packaged nuts reaching the customers.
-
Streamline Recruitment With Confidence
Transform your clinical trial recruitment with a service that delivers faster, more cost-effective enrollment, particularly for hard-to-reach populations.
-
Optimizing Local Affiliate PV Operations
Discover how a global biopharmaceutical company partnered with PharmaLex to implement a streamlined, standardized model for international ICSR processing, compliance, and cross-functional collaboration.
-
A Paperless Lab Simplifies, Accelerates Full Sample Lifecycle Data Management
Implementing IDBS’ ELN helped Crown Bioscience realize electronic experimental records, optimizing our business processes and helping them to be more standardized.
-
Solid-State Characterization Of A Small Molecule API
Ensuring the consistency and purity of the API is paramount when developing a new drug. Learn about the importance of CRO partnerships when facing tight deadlines and complex scientific hurdles.
-
Enhancing Preclinical Cell Therapy Development
Automation in cell therapy development boosts reproducibility, reduces error, and enhances yield. Learn how it’s reshaping preclinical workflows and accelerating clinical trial readiness.
-
California's First Installation Of 2nd Generation ATAD
Calera Creek Water Recycling Plant, located in Pacifica, California, recently retrofitted to the Thermal Process Systems’ ThermAer™. This is California’s first installation of the second generation ATAD system.
-
How Strategic Valve Installation Prevented Service Disruption at a Senior Living Facility
Learn how Hydra-Stop’s insertion valve provided new control points to mitigate future disruptions during a service line emergency.
-
Improving Patient Health And Safety: Medical Affairs
A medical affairs department needed leaders with scientific and project management expertise. With no time for training or risk tolerance, a consultant was hired to align activities with development and launch plans.
-
Symmetry Surgical Uses Software To Enhance Quality
Symmetry Surgical enhances operations and quality management using software to improve efficiency, compliance, and data-driven decisions while producing over 2 million surgical devices annually.
NEWS
-
Baden-Württemberg's Largest Solar Park Now In Operation5/28/2025
EnBW has commissioned its solar park in Langenenslingen-Wilflingen (district of Biberach in the southwest of Germany).
-
SJW Infrastructure Improvement Progressing At Cambrian Tank Replacement Project5/29/2025
San Jose Water (“SJW” or “the Company”), a wholly owned subsidiary of H2O America, has made meaningful progress on its Cambrian Tank Replacement project, a major investment in the long-term reliability and seismic resilience of the Company’s water system.
-
Smarter Dentistry Starts Here: Muskan.AI Enhances Diagnostics, Imaging & Patient Care5/19/2025
Muskan.AI, a pioneer in the application of artificial intelligence to dentistry, has reached several key milestones in its mission to transform dental care through intelligent, accessible, and clinician-focused technology.
-
Aircraft Toilets Could Flush Out Spread Of Global Superbugs8/18/2025
Wastewater from aircraft toilets could provide a critical warning system for the global spread of antimicrobial resistant (AMR) superbugs, a silent pandemic that threatens to kill more people than cancer by 2050.
-
Stoichiometric Crystal Shows Promise In Quantum Memory7/7/2025
For over two decades, physicists have been working toward implementing quantum light storage—also known as quantum memory—in various matter systems.