Accelerate Your Operations With Smarter Trial Management
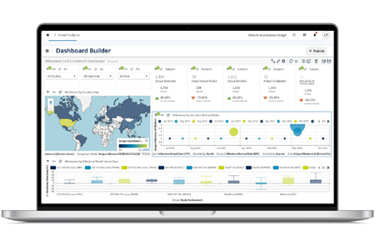
Medidata CTMS is a cloud-based solution that centralizes clinical and operational data to improve the efficiency and oversight of clinical trials. By replacing manual spreadsheets with an intuitive interface, it minimizes user effort and automates notifications for key events. The system accommodates both sponsor-led and CRO-managed studies, providing flexibility and control. Its scalable architecture ensures reliable performance and supports growth. Key functionalities include monitoring visits, issue management, oversight reporting, study management, document tracking, and informed consent form (ICF) review. This platform's unified data architecture allows for single data entry, enabling seamless use across all applications and streamlining trial operations. Medidata's Professional Services enhance the system's value by offering extensive support beyond initial implementation.
Get unlimited access to:
Enter your credentials below to log in. Not yet a member of Clinical Leader? Subscribe today.
