Begin Your AI-Led Digital Clinical Transformation With A Phased Approach
By Krishnan Rajagopalan, Ph.D., president, Life Sciences – Strategic Advisory and Consultancy Services

AI and generative AI are becoming a cornerstone of every enterprise strategy, but many organizations are finding that implementing holistic AI at scale for sustainable benefits is easier said than done. In 2025, enterprise AI adoption is at an all-time high, with surveys showing that over 80% of companies are using or exploring AI. However, only a small percentage of companies are beginning to deliver meaningfully on their promise — and many are continuing to succumb to “death by a thousand pilots.”1
Organizations need to establish a holistic AI-led digital strategy, starting with a top-down, phased approach, complemented by strategic workforce planning (WSP) to capture value generated from AI transformation. In this first article, we review potential approaches for phased implementation, using clinical trials as an example.
Helpful to this endeavor is understanding this Digital Clinical Maturity Assessment (DCTMa) framework , which comprehensive across 10 core parameters for implementing holistic AI transformation.2 The approach allows organizations to systematically analyze their current maturity along these parameters (“current score” in the illustration below). They can also benchmark the scores on the key parameters with industry peers, identify gaps, and develop their AI-led digital clinical transformation strategy and implementation plans. Here, we have provided an approach to implement the strategy in a phased manner.
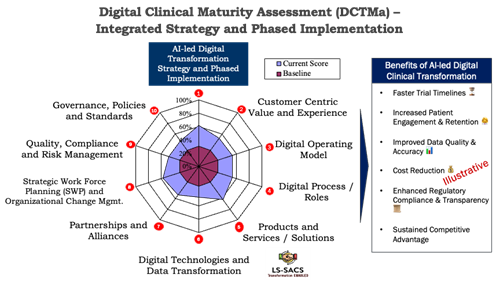
How To Phase Your AI Transformation
AI-led transformation requires a holistic strategy that encompasses multiple topics or levers to achieve most of the transformation objectives. Some potential KPIs (with examples for clinical trials) can be developed using the balanced scorecard (BSC). A typical BSC provides a balanced approach for most of the KPIs along four groups and assigns a weight based on a company’s own vision/plans, goals, and competitive positioning:
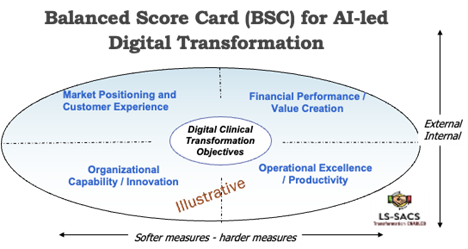
- Market Positioning and Customer Experience: This ensures that AI directly enhances customer satisfaction and increases their loyalty to the brand and increases the value of the customer base. Potential KPIs can include AI impacted net promoter score (NPS), customer lifetime value (CLV) uplift, and impact of digital clinical trials (DCTs) on new product launch timelines/approvals
- Financial Performance/Value Creation: This quadrant focuses on return on AI investment (ROAI). Potential KPIs can include, net financial gain (cost savings + revenue uplift) from AI portfolio of investments, percentage of digital revenue contribution, and revenue gain due to faster time to market from AI-driven digital clinical trials
- Organizational Capability/Innovation: This quadrant focuses on employee / staff skill level development and AI tool adoption rate. Potential KPIs can include time to-market for new AI features, percentage of of AI models audited for bias, percentage of eligible employees using AI for processes, and percentage of staff trained to be part of AI initiatives
- Operational Excellence/Productivity: To ensure most activities are conducted using digital means and ensure tracking and monitoring of the progress of these initiatives. Potential KPIs can include selected process cycle time reduction, percentage of high-value tasks automated and AI model accuracy/F1 score, and percentage reduction in trial time or patient recruitment
Given the difficulties in realizing tangible ROI from a complex AI transformation strategy, life sciences companies must develop a phased implementation plan along with the initiatives, timelines, and KPIs to track the success of each of the initiatives and their alignment with the overall strategy. Such a plan needs to include the key roles and responsibilities to manage complex changes effectively, minimize risk, secure stakeholder buy-in, and maximize long-term benefits.
Phase 1: Begin With Foundational AI And Robotic Process Automation (RPA)
- Definition/Scope: Robotic process automation (RPA), workflow automation, scripting and macros, and basic integration platforms
- Capabilities, Technology, And Tools: Task execution without cognition, structured input to structured output, no decision-making and rule-following only. Technology platforms include Zapier, UiPath, and Power Automate.
- Examples: Automatically moving files between systems, pre-scheduling patient visits
- Estimated Benefits: 20%–40% time savings; 15%–30% cost savings, based on client experiences
Strategic activities and planning during Phase 1 aim to establish a solid data and infrastructure foundation, automate repetitive tasks, and achieve tangible ROI through efficiency gains. Key activities include:
- Data infrastructure and governance setup
- RPA implementation
- Level 1 conversational AI and predictive analytics
- Rigorous performance tracking and measurement of ROI (aligned to strategy)
- Start training/retooling staff to pick up Phase 2 activities
Expected outcomes include:
- Reduced cost of selected activities/processes
- Increased efficiency and compliance through faster processing times and improved accuracy/quality
Some illustrative examples in clinical trials and operations for this phase on automating repetitive, rules-based tasks using traditional RPA, combined with basic ML to handle structured data, include:
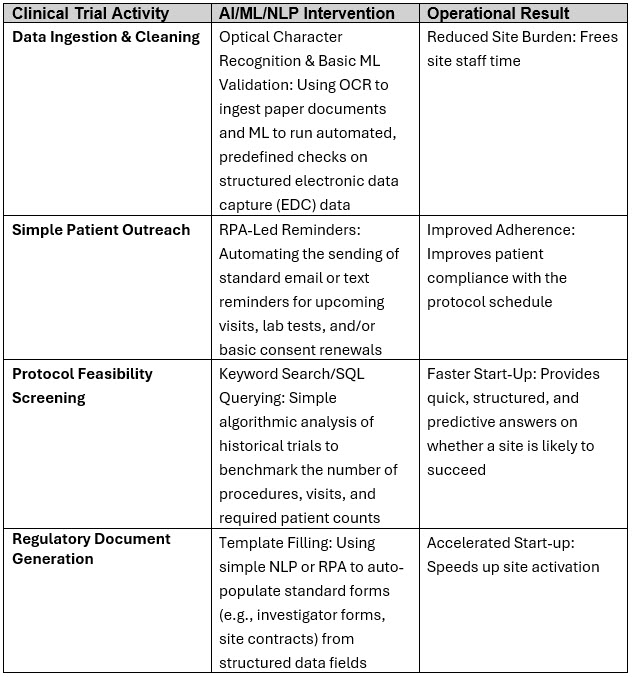
These tangible metrics will improve employee/workforce morale, allowing them to focus on higher-value activities and be prepared for Phases 2 and 3.
Phase 2: Add Autonomous AI And Intelligent Process Automation (IPA)
- Definition/Scope: Use large language models (LLMs) or generative AI to assist in content creation, summarization, reasoning, and knowledge tasks
- Capabilities, Technology, And Tools: Understand natural language, generate and summarize content, and support reasoning over unstructured data. Resources include ChatGPT, Claude, Gemini, LLaMA, and generative AI for text, images, code, NLP, NLG, and document understanding
- Examples: Drafting clinical study reports, summarizing adverse event narratives, generating scripts or documentation in IT, creating de novo drug molecule structures
- Estimated Benefits: 40%–70% reduction in knowledge task effort, two to four times productivity boost, and enables human-in-the-loop acceleration, based on client experiences
Phase 2 focuses on implementing AI systems that can operate with minimal human intervention, augment human decision-making, and create personalized experiences. Extending and expanding from RPA to IPA will be a key focus during Phase 2. Key activities include:
- More advanced predictive and prescriptive analytics
- IPA and autonomous workflows
- Personalized user experiences and AI-assisted design
Expected outcomes include:
- Better decision-making through AI-driven insights provides real-time and proactive strategic choices
- Improved customer satisfaction and optimized operations provide competitive advantages
- Increased agility to adapt to market changes and operational demands
- Increased staff focus on more strategic activities and the use of AI to develop new products and services
These outcomes will ensure continued improvement of employee/workforce morale, as well as the upskilling of AI and digital maturity through Phase 2.
Some illustrative examples in clinical trials and operations for the Phase 2 leveraging of advanced ML and NLP to handle unstructured data, provide insights, and augment human decision-making are provided below:
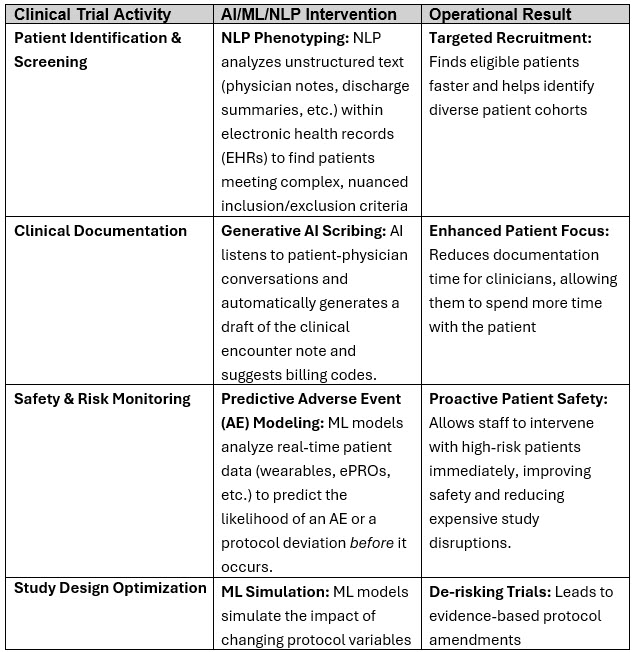
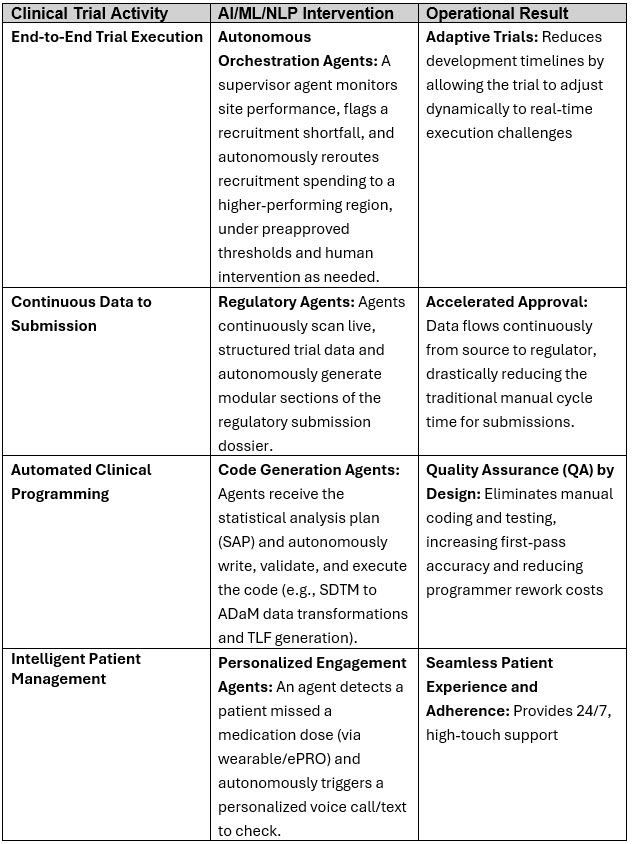
Phase 3: Embrace Agentic AI And Holistic Transformation Initiatives
- Definition/Scope: AI agents that can plan, reason, and execute multistep tasks autonomously using tools, memory, and feedback.
- Capabilities, Technology, And Tools: Goal setting and sub-task planning, learning and iterating based on feedback. Agent frameworks include auto-GPT, LangChain Agents, CrewAI, Tool-chaining, as well as multi-agent orchestration, memory, and long-context LLMs.
- Examples: Autonomous trial monitoring agent, self-healing infrastructure in IT, end-to-end medical document generation, closed-loop compound optimization in drug discovery
- Estimated Benefits: 70%–90% automation of complex workflows, real-time adaptive decision-making, five to 10 times operational throughput, based on client experiences and SME feedback
Focus in this phase will be on implementing highly autonomous AI agents that can define, plan, execute, and adapt their goals, leading to significant strategic shifts and new business models. These can be aligned with the AI-led strategic organizational transformation desired by leadership.
Key activities include:
- Agentic AI pilots customized for specific functional domains
- Establish ethical AI and governance for agentic systems
- Enterprise-wide agentic integration and new business models
- More advanced predictive and prescriptive analytics
Expected outcomes include:
- Reshaped market position and new revenue streams
- Levels of operational efficiency previously thought to be impossible are achieved
- Individualized staff/customer (patient) experiences are delivered
- AI-driven discovery and rapid development of new solutions
- Self-optimizing systems that can adapt to unforeseen challenges
Many of these outcomes will move the company into a mature AI-driven and agile organization that has improved its EBITDA, competitive position, and AI-savvy workforce.
Potential examples in clinical trials and operations for the Phase 3 include fully autonomous AI agents that can reason, plan, execute multistep tasks, and interact with multiple systems independently. How well these activities translate into implementation in Phase 3 will depend on the success of Phases 1 and 2 and if the basic processes, skills, and technology changes were implemented.
Key Benefits Of A Phased AI Strategy
1. Validation of the AI-driven strategy and the desired strategic outcomes
For novel AI applications with key business goals, a phased approach provides crucial opportunities to validate the business model with real-world/market feedback.
- Tests with real data: By testing components with real data in a controlled environment, an organization can ensure the AI solution is aligned with both technical requirements and business goals.
- Aligns with strategic objectives/KPIs, including financial goals: Measuring KPIs from the BSC at each phase provides greater insight into how the AI initiatives contribute to the bottom line, ensuring efforts remain focused on delivering value and on the opportunity to refine objectives and initiatives.
2. Ability to ensure stakeholder buy-in and manage change
AI transformation involves significant internal organizational and cultural, as well as external customer and regulatory, shifts. A gradual approach helps manage these changes more effectively.
- Fosters adoption: Employees are given time to adapt to new systems and personal KPIs, easing the learning curve and reducing resistance to change.
- Demonstrates value: Tangible benefits are delivered to both internal and external customers in early stages through quick wins, building momentum, and proving the AI's value to all stakeholders and employees.
3. Efficient resource allocation, training, and re-skilling match phased AI implementation initiatives
A phased rollout enables better planning and allocation of resources, including recruitment, training, budget, and time.
- Spreads out costs: The financial investment is spread over time, reducing the significant up-front costs and budget risks associated with a large-scale project.
- Prevents bottlenecks: Project teams can focus on a single component at a time, ensuring that resources and support are not stretched thin across the organization.
4. Continuous learning and improvement
A phased strategy treats AI implementation as a continuous strategic program, not a one-time event, allowing for ongoing refinement.
- Incorporates feedback: Each phase allows for the collection of user feedback, which can be incorporated into subsequent iterations to improve the AI model's accuracy, relevance, and benefits.
- Fosters agility: The iterative nature of a phased approach provides the flexibility to adapt to new developments in AI technology and evolving business, market, and customer/patient needs.
5. Proactive risk-mitigation
By breaking down AI implementation into smaller stages managed by a robust governance structure, you can identify and address problems before they cause large-scale failure.
- Controlled environment: Pilot projects provide a low-stakes way to test the AI's performance, refine the technology, and measure success against KPIs.
- Contained disruptions: If an issue or bug arises, its impact is limited to a smaller, isolated area of the business instead of affecting the entire organization, customer services, and/or the brand.
Life sciences organizations need to develop a holistic approach to their AI-led digital transformation strategy, document desired outcomes and metrics (e.g., balanced scorecard), and communicate to the full organization. The strategy should be accompanied by a detailed phased implementation plan with the planned initiatives, milestones, and KPIs. To ensure successful implementation, the plans should cover the processes, staff/skills, technology/tools, and budgets required. Integrated strategic workforce planning (SWP) that is customized to fit the evolving AI-led digital operating model — recruiting, training, retraining, and performance metrics — is also critical for the overall success of the program.
References:
- https://www.mckinsey.com/capabilities/mckinsey-digital/our-insights/rewiring-for-the-era-of-gen-ai
- How To Accelerate Digital Clinical Transformation Through A Holistic Approach
About The Author:
 Krishnan Rajagopalan, Ph.D., is the president of Life Sciences – Sourcing Advisory and Consultancy Services and brings 30 years of experience in management consulting and advisory, strategic sourcing and outsourcing, and global services delivery to Fortune 100 clients. He has advised small, medium, and large life sciences clients and digital/SaaS product companies on growth strategy, organizational change and communications, operational model design and implementation, strategic and sourcing and outsourcing (CRO, BPO, FSP and IT services). Krishnan also delivered full-service CRO, CDM, pharmacovigilance/safety, biostatistics/programming, MW, regulatory affairs, commercial services, and IT/digital to more than 100+ life sciences companies through Fortune 500 global services providers from India, China, Argentina, Europe, and the U.S. He can be reached via email, by phone at +1 908 380 3343, and on LinkedIn.
Krishnan Rajagopalan, Ph.D., is the president of Life Sciences – Sourcing Advisory and Consultancy Services and brings 30 years of experience in management consulting and advisory, strategic sourcing and outsourcing, and global services delivery to Fortune 100 clients. He has advised small, medium, and large life sciences clients and digital/SaaS product companies on growth strategy, organizational change and communications, operational model design and implementation, strategic and sourcing and outsourcing (CRO, BPO, FSP and IT services). Krishnan also delivered full-service CRO, CDM, pharmacovigilance/safety, biostatistics/programming, MW, regulatory affairs, commercial services, and IT/digital to more than 100+ life sciences companies through Fortune 500 global services providers from India, China, Argentina, Europe, and the U.S. He can be reached via email, by phone at +1 908 380 3343, and on LinkedIn.
