CRO Outsourcing — How To Optimize Your Selection Process
By Joelle Herman, president, NeoTrials, LLC

This is part 2 of a three-part article series on how best to procure, manage, and implement best practices in the complicated CRO market.
In part 1, types of procurement and strategic sourcing were discussed, showing how judicious planning and various selection criteria support a best fit outsourcing approach to your trial(s). So, what happens now? The selection committee will formalize the details using a request for proposal (RFP). The RFP is where you define and finalize the scope of services you are looking to outsource, and this holy grail of documents can make all the difference in a fair and equal comparison of vendors. Whether you put the information in a Word document or Excel file, or have an online portal, the key is to be able to compare apples to apples.
Several previous Clinical Leader articles outline the basic details needed, so the focus of this article will provide practical advice to ensure sponsors have thought through all the “what ifs.”
RFP Workflows
The sponsor workflow from planning through selection can be arduous and time consuming, but thoughtful details of your workflow and due dates will only make for a better procurement. The selection committee must allow for reasonable deadlines for each step of the process to receive highly competitive proposals on time. Typically, contract research organizations (CROs) need 10 to 20 business days to do a good job; anything less will result in boilerplate documents that don’t allow for a best fit assessment. Once all the key tasks below are put into a timeline/Gantt chart or just assigned due dates, the RFP can be put together and the process commenced. Each step may have CRO deadlines delineated in the RFP document. For example, if you allow CROs to send questions or clarification requests before the final bid is received, you should provide a deadline for sponsor receipt of all questions and when and how the answers will be provided. A fair competition provides all anonymous Q&As to all bidders in the same time frame. Often, allowing five to seven days to review the RFP will foster thoughtful comments or insightful questions that may even cause you to revise a specification. This back and forth not only allows higher quality bids but creates initial collaboration to mitigate common development risks and challenges. Basic steps in the RFP process may look like this:
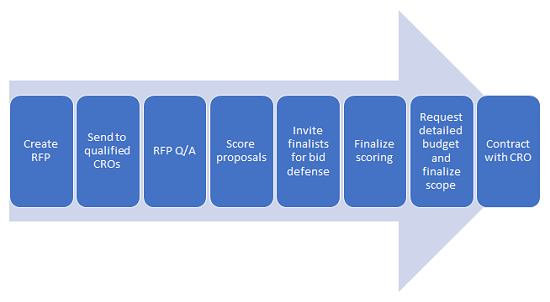
Sponsors that understand all sides of the process typically are better able to reduce future challenges with CRO management. An example of the CRO’s workflow1 and proposal management process (below) shows that the process can be a heavy lift to put together a competitive package.
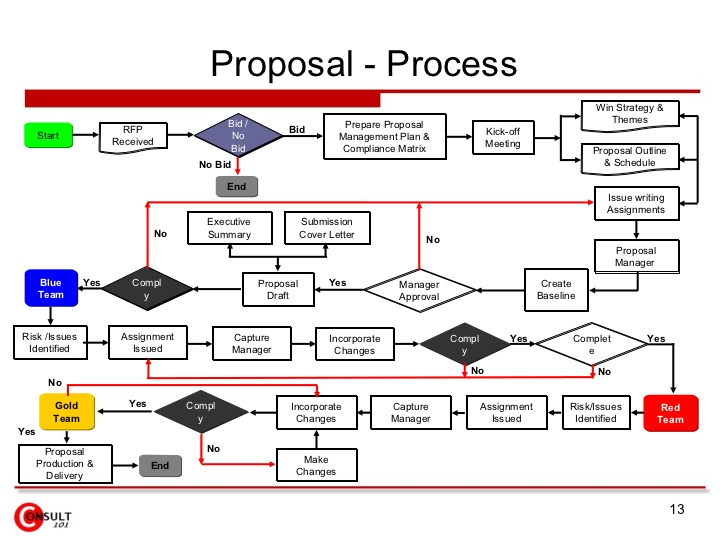
Thinking about what you want to receive from CROs/vendors and having an agreed approach will also minimize questions and allow for thoughtful responses. The following checklist will help you get started.
RFP Documentation Checklist
Minimum details to provide in your RFP are as follows:
- Background
- Proposal guidelines/deadlines and forms (include Intent to Respond form)
- Proposal deliverables
- Protocol/protocol synopsis
- Program/project timelines/milestones/assumptions
- Roles and responsibilities
- Technical specifications for each area of service (i.e., detailed tasks for project management [PM], clinical operations, data management [DM], biostatistics, pharmacovigilance, etc.) and the proposed team (including CVs) and vendors/third-party subcontractors proposed
- Specialty experience or past performance requested/required (unique qualifiers)
- Important details, challenges identified by sponsor team and CRO recommendations
- Simple budget template (high level to be able to compare without increasing CRO burden)
- Evaluation criteria (financial and technical)
The details sponsors provide not only add context, history, expectations, and challenges, but also allow CROs to be creative to win the bid.
Additional means to differentiate candidates can be achieved through a request for information. This is often done prior to this step, but if your organization has not yet prequalified a CRO/vendor, now is the time to get some key information, such as the following:
- Organizational chart/office locations and services at each site
- Financial stability
- Insurance
- Compliance with business ethics/privacy
- Facilities and computer management systems
- Quality management systems (including documentation management)
- Human resources and training
- Project management (including SOPs and third-party vendor management)
- Specialty services (electronic data capture [EDC], lab, imaging, interactive technologies, etc.)
Strategic contracting2 can also be a differentiator. When you have several unknowns, the risk can be balanced with the type of contract agreed upon. As the sponsor, ask yourselves what kind of contract you will accept to minimize the risk of overruns if the plan doesn’t get executed the way you predicted. Trial-based contract structures include milestone payment, cost plus incentive fees, time and materials/unit price, or a hybrid model.
Using a “day in the life of” (DILO) or simulations3 can be one of the best approaches for a bid defense meeting. Ultimately, you want to see certain operational tasks and how they will add value to your program. This is a great way to see opportunities and risks. Some examples of this include walking through patient introduction to the study and to various visits; simulating a device/technology to be used; and performing a data point DILO – a mockup of the EDC or interactive web response.
A best practice once all the proposals have been received and reviewed is to have a consistent process for evaluating the bid.The RFP checklist provides for criteria, and bidders should know what is important to your selection. Evaluation criteria or measures that are associated with standards for your scorecard can be assigned weights and scored.
Example Sections of Proposal Scorecard
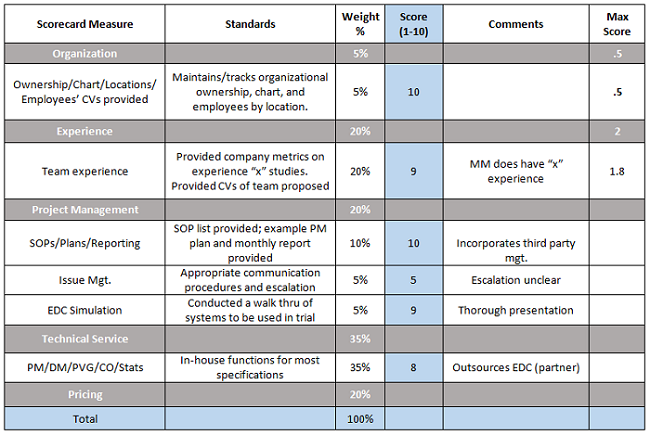
It is easy to forget information that was also part of the bid defense discussions, but fair scoring provides for an assessment based on the information provided and demonstrated, not promised verbally. Once the scope of services or any revisions are requested and provided by the winning bidder, you are ready to contract with the CRO.
Regulations
In contracting with the CRO, sponsors have to consider regulatory and legal implications. The U.S. FDA and global country regulations afford sponsors the opportunity to transfer regulatory obligations to a CRO. Under the U.S. Department of Health and Human Services, the FDA Code of Federal Regulations, 21 CFR Part 312.52,4 gives sponsors the ability to transfer any or all obligations of drug research. Device manufacturers don’t have such a clear-cut subchapter, but documenting any agreed transfer is vital to both parties. A good RFP and procurement process will stipulate these expectations and many CROs may provide detailed documentation of such a transfer, if they don’t create one based on roles and responsibilities and ensure your regulatory attorney/legal counsel has reviewed it. Regulatory risk and a sponsor’s focus on regulatory compliance and CRO reliability go hand in hand in the final selection of a CRO partner.
The third and final article in this series will cover common challenges and best practices in CRO outsourcing.
References:
- https://www.slideshare.net/anandsubramaniam/proposal-management-process-2755455
- https://www.simplilearn.com/project-management-contracts-article
- https://www.clinicalleader.com/doc/a-simulation-based-approach-to-cro-selection-0001
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRsearch.cfm?CFRPart=312
About the Author:
 Joelle Herman is the president of NeoTrials, LLC and brings over 20 years of experience in clinical research. Her expertise is in clinical operations program management, focused on outsourcing management. She created NeoTrials after years of consulting to small biopharma and other consulting firms. Prior to consulting, she served as director of clinical operations at DynPort Vaccine Company LLC. Earlier in her career, Herman led global clinical programs for organizations such as Oxxon Therapeutics/Oxford BioMedica, AAI Pharma, Antigenics Inc., and Apheresis Technologies, Inc. She has conducted and managed over 30 global trials in drugs, devices and vaccines including well-known products. Herman holds a master of public health in epidemiology and a bachelor of science in microbiology, both from the University of South Florida, and is a Certified PMI Project Management Professional. Connect with her on LinkedIn
Joelle Herman is the president of NeoTrials, LLC and brings over 20 years of experience in clinical research. Her expertise is in clinical operations program management, focused on outsourcing management. She created NeoTrials after years of consulting to small biopharma and other consulting firms. Prior to consulting, she served as director of clinical operations at DynPort Vaccine Company LLC. Earlier in her career, Herman led global clinical programs for organizations such as Oxxon Therapeutics/Oxford BioMedica, AAI Pharma, Antigenics Inc., and Apheresis Technologies, Inc. She has conducted and managed over 30 global trials in drugs, devices and vaccines including well-known products. Herman holds a master of public health in epidemiology and a bachelor of science in microbiology, both from the University of South Florida, and is a Certified PMI Project Management Professional. Connect with her on LinkedIn
