Establishing TMF Quality Goals For Greater Trial Efficiency
By Ken Keefer, Keefer Consulting

Does your clinical development group rely on its TMF as a resource for designing and conducting trials efficiently? Would maintaining timeliness in filing complete, ALCOA-compliant documents to the TMF be enough if efficiency were a goal of every clinical trial?
Sponsors may want to consider these questions when planning for the ICH Harmonised Guideline for Good Clinical Practice (GCP), E6(R3). The current draft guidance proposes principles “intended to support efficient approaches to trial design and conduct” as well as “to protect the rights, safety and well-being of participants and assure the reliability of results.” E6(R3) states that to accomplish these goals, “Quality by design should be implemented to identify the factors (i.e., data and processes) that are critical to ensuring trial quality and the risks that threaten the integrity of those factors”.1
Quality by design (QbD) could be key to establishing TMF processes that provide study managers with ongoing, current information about factors critical to trial quality (CTQs), thereby contributing to E6(R3) compliance and perhaps accelerating approvals.2
J. M. Juran developed a “quality planning road map” three decades ago that identifies all customers of a product or service, determines their CTQs, and plans for the design and development of product or service features to meet customer needs.3 Step 1 on the road map is to establish quality goals. Applying this step to TMF process development could help transform the TMF into the role E6(R3) envisions for it:
“The sponsor and investigator/institution should ensure that the essential records are collected and filed in a timely manner, including those required to be in place prior to the trial start, which can greatly assist in the successful management of a trial.”
Does Your TMF Assist In Successful Trial Management?
Imagine that a study manager requests a copy of a document directly from its author instead of accessing it in the TMF. If asked why, the manager may reply that going directly to the author ensures the copy is of the most recent version.
Lags in filing to the TMF => an incomplete TMF that cannot fully support decision-making.
How could this happen in spite of ongoing efforts by TMF managers and specialists to collaborate with different functional units across clinical development to ensure timely filing of documents? QbD as emphasized by E6(R3) may help answer this question and point to refinements of current processes that would improve timeliness in collecting and filing essential records.
Quality planning works best when quality goals based on business objectives are set at the top and propagated throughout the organization. QbD is a means for collaborating cross-functionally to set departmental quality goals in line with those set by upper management. Planning TMF quality and trial quality at the same time is an application of this principle. Applying the Juran quality planning road map to TMF process redesign would examine the needs of all current and potential customers of TMF services, including those responsible for conducting trials and the suppliers of content documenting those trials.
Establishing Quality Goals
Quality planning is a process of establishing quality goals for designing a product or service and the processes and controls necessary to produce, deliver, and maintain it.
Quality Goals And Business Objectives
Quality goals support strategic and tactical business goals at all levels of the organization. For example, a sponsor sets a strategic goal to reduce its costs of poor quality while accelerating product approvals. A possible tactical goal would be to enable study managers to view documents approved and held by a CRO. A TMF service feature might be an interface for viewing approved documents in the CRO’s TMF. Processes supporting this feature could include a workflow and standard operating procedure (SOP). A process control might be an alert to the sponsor if the CRO fails to meet its service level agreement for timeliness in making an approved document visible.
Origins Of Goals
TMF quality goals originate from various sources. For example, public demand for faster development of affordable therapies to reduce the societal impact of disease may spur regulatory authorities to adopt E6(R3) and promote greater efficiency.
Quality goals may originate from management and focus on the enterprise or on smaller business units. A goal of achieving E6(R3) compliance would typically originate from upper management and extend throughout the organization. A single department or group of related departments could undertake such an initiative on its own, although that would be less likely to influence other parts of the organization.
Many TMF departments already set specific benchmarks measuring quality, completeness, and timeliness. Employing QbD to establish TMF quality goals supporting those set by upper management might require expanding this scope to include additional functional units that either provide or consume TMF content.
Information technology and data standards can be sources of quality goals. Cloud-based services like eTMFs offer many features affecting quality, such as workflows to facilitate document approvals. The Clinical Data Interchange Standards Consortium (CDISC) has assumed control of the TMF Reference Model and is developing it as a standard for storing TMF content and transporting it between systems.
Customers
Every origin of a quality goal involves customer needs. When efficiency is a goal of a trial, content stored in the TMF must serve internal customers within clinical operations, as well as external customers such as regulatory agencies.
eTMF products, internal processes, and SOPs are geared primarily to satisfy regulatory customer requirements. Adaptation will be necessary as regulators increasingly view the TMF as consisting of all content essential to understanding the history of a trial, regardless of what systems contain that content. Defining TMF completeness in this way promises to aid investigators in assessing GCP compliance and also to supply internal customers with information relevant to assessing progress and making decisions.
Study Design And TMF Quality
Coordinating interactions between study design and TMF service design promotes efficiency. E6(R3) requires sponsors to base study designs on good information, much of which lies in documents created before or during the study design process. Fully documenting trial history requires adding documents to the TMF when first referenced during study design or created as a by-product of the study design process. Synchronizing TMF quality planning with trial quality planning can support the creation of a TMF capable of providing current trial-related information beginning with pretrial activity.
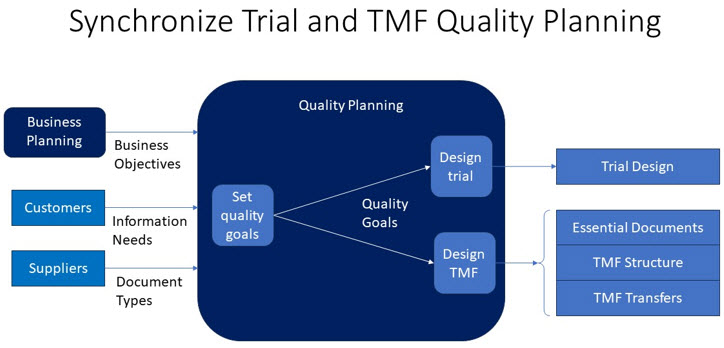
Quality Measurement And TMF Completeness
The Trial Master File Plan Template is a de facto industry standard for planning TMF quality and has been administered as a resource supporting the CDISC TMF Reference Model.4 It describes how a “TMF that is high quality, complete and real-time ready will be created, maintained, closed, and archived” and outlines the roles responsible for executing the TMF plan. Measurement of TMF quality as widely practiced focuses on three goals embedded in this description: quality, completeness, and timeliness. Measuring data integrity is also important as eTMFs rely on metadata to describe and locate content.
Transporting content and metadata into a TMF from other systems can be critical to its completeness. Yet this is often neglected until end-of-study, resulting in a TMF that is not always “real-time ready.” The industry must solve this problem to satisfy regulators’ expectations of full access to TMF content.
Section 11 of the TMF Plan, Transfer and Archival of TMF, lists information to be included in a TMF transfer agreement between sending and receiving organizations. The eTMF Exchange Mechanism Standard (EMS), based on the TMF Reference Model, specifies a similar agreement for exchanging TMF content and metadata between systems. Industry adoption of a TMF content transport standard will hopefully grow as the TMF Reference Model and EMS are incorporated into the CDISC family of standards.5
Your TMF As A Trial Management Tool
Sponsors can begin now to design more efficient TMF services and prepare for regulatory implementation of E6(R3). Achieving E6(R3) compliance resulting in greater trial efficiency will take years for many organizations. The more successful transitions will benefit from top management commitment that extends to all parts of the organization, including clinical and regulatory operations.
Strive for upper management commitment to strategic quality goals based on business objectives. Break strategic quality goals into quantifiable, time-constrained subgoals for deployment to lower-level organizational units. Assure sufficient time and funds for projects to implement quality goals.
Optimize the trial management processes across related multiple functions, including study design and TMF services. Optimizing separate autonomous functions tends to suboptimize related multifunctional processes.
TMF Completeness And Interoperability
Many organizations measure TMF completeness as documents received versus documents expected and refine processes as needed to improve this ratio. More could be done to improve TMF completeness by designing processes to make all essential documents visible, regardless of the systems in which they reside.
Establish quality goals for multi-system interoperability. Tell your eTMF vendor that your organization wants to exchange TMF content with other vendors’ systems in near real time. Participate in efforts by industry organizations such as CDISC to develop TMF content and metadata exchange standards that all vendors can adopt.
Sustaining Momentum
Gaining cooperation among multiple functional units requires management support and is key to sustaining TMF quality improvement. At minimum, clinical operations management should be onboard. Tying project proposals to quality goals can improve the odds of funding a quality improvement program.
Management support is strengthened by an awareness of the costs of poor quality. Incomplete provision of TMF content and metadata can lead to inspection findings, operational delays, and errors in trial conduct.
Management must acknowledge the costs of maintaining continuous quality improvement, including:
- quality training for planners,
- quality planning projects,
- setup for TMF content and metadata exchanges,
- administration, and
- opportunity cost of investing in cumulative efficiency vs. completing active trials quickly.
Conclusions
Establishing TMF quality goals is the first step in applying QbD to improve TMF services. Planning TMF quality in collaboration with the study design process could produce TMFs capable of realizing the E6(R3) vision of “assist[ing] in successful trial management” from the outset of pretrial activity.
TMF quality goals should be traceable to business objectives, including those that address regulatory change and capitalize on evolving information technology. Designing and sustaining the TMF as a trial management tool requires management commitment, ideally from the top. Sustaining TMF quality requires acknowledgement of the costs of poor quality and of continuous quality improvement.
References:
- International Council For Harmonisation Of Technical Requirements For Pharmaceuticals For Human Use, ICH Harmonised Guideline Good Clinical Practice (GCP) E6(R3) Draft version, ICH, 19 May 2023, https://database.ich.org/sites/default/files/ICH_E6%28R3%29_DraftGuideline_2023_0519.pdf.
- Ken Keefer, Designing Quality Into Your TMF: ICH E6(R3) And Advancing Trial Efficiency, 17 Jul 2023, https://www.clinicalleader.com/doc/designing-quality-into-your-tmf-ich-e-r-and-advancing-trial-efficiency-0001.
- J. M. Juran, Juran on Quality by Design, 1992, New York, The Free Press.
- TMF-Plan-Template-v2-2022-10-21, TMF Reference Model, 21 Oct 2022, https://tmfrefmodel.com/download/4789/?tmstv=1667384522, retrieved 21 Jun 2023.
- Ken Keefer, TMF Reference Model Affiliation With CDISC Could Improve TMF Interoperability, 20 Mar 2023, https://www.clinicalleader.com/doc/tmf-reference-model-affiliation-with-cdisc-could-improve-tmf-interoperability-0001.
About The Author:
 Ken Keefer founded Keefer Consulting Inc. early in his career with a commitment to helping clients develop innovative computer-based solutions to business problems. Today, he applies his experience to transforming clinical operations through improved system interoperability and process efficiency. He envisions a world in which clinical operations can share information seamlessly with regulators, service providers, and partners through common standards and processes. The ultimate goal is to achieve positive outcomes for patients quickly and cost-effectively.
Ken Keefer founded Keefer Consulting Inc. early in his career with a commitment to helping clients develop innovative computer-based solutions to business problems. Today, he applies his experience to transforming clinical operations through improved system interoperability and process efficiency. He envisions a world in which clinical operations can share information seamlessly with regulators, service providers, and partners through common standards and processes. The ultimate goal is to achieve positive outcomes for patients quickly and cost-effectively.
Ken holds an MBA from Temple University and a post-graduate certificate in pharmaceutical and healthcare business from the University of the Sciences in Philadelphia. Ken can be reached at kkeefer@keeferconsulting.com.
