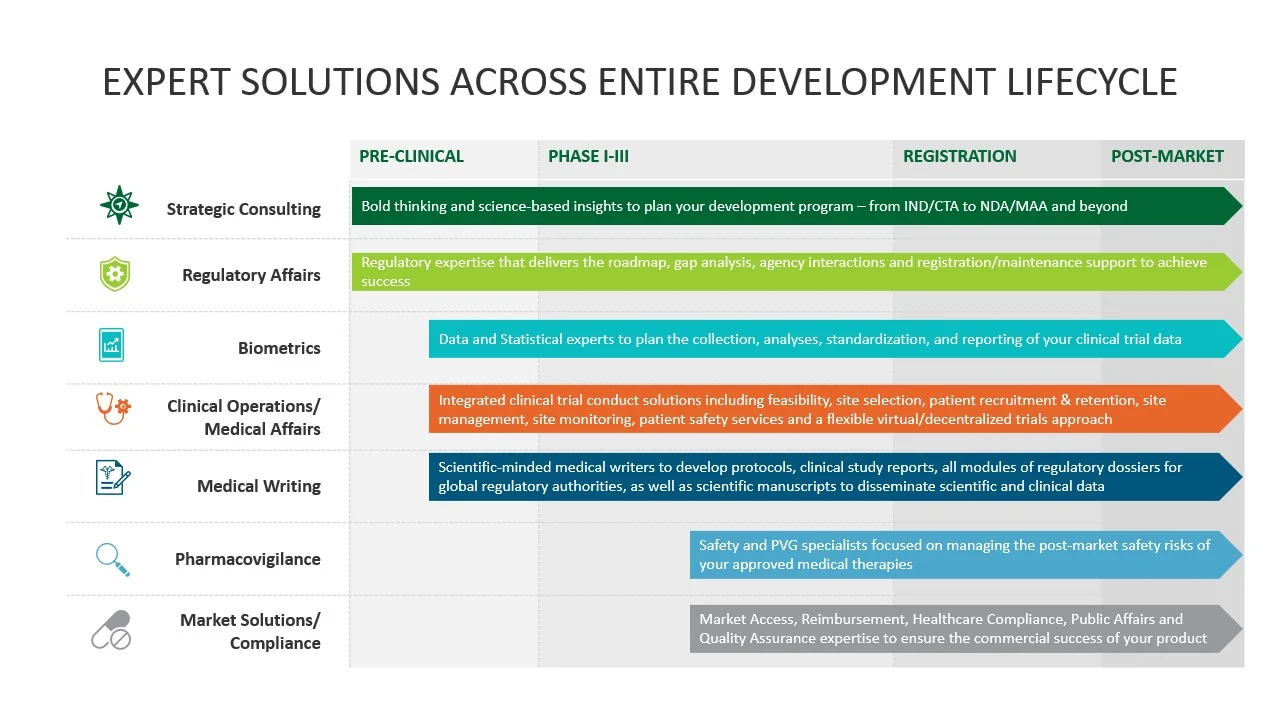
Full Service Clinical Development Solutions

Getting a novel therapy through the clinical development process to approval is complicated. Unearth how to advance novel medical therapies from Phase I-III clinical development to market with confidence.
Advance your novel medical therapies from Phase I-III clinical development to market with confidence.
Getting a novel therapy through the clinical development process to approval and commercialization is complicated and full of challenges. Veristat has assembled an extraordinary team of scientific-minded experts who strategically design and execute clinical trials and prepare your clinical data for regulatory review. Our integrated regulatory, clinical, biometrics, safety, and medical writing& experts support small to mid-sized biopharma companies running their development programs throughout North America, Europe, and many other regions worldwide.
Helping you make and implement the right decisions at the right time is our strength. We help you overcome your challenges and advance your compound to the next step in the clinical development process.
Veristat teams supported marketing applications for 8 therapies that received regulatory approval in 2022, mostly for therapies designed to treat rare diseases.
Navigating the Clinical Development Journey | Phase I-IV
Avoid Unknowns from the Start
We are here to establish patient safety throughout clinical trial planning and conduct, navigating the regulatory approval process, and post-marketing surveillance. When you start thinking about your first-in-human trials or have solved all the complex challenges and do not know how to move forward, it's time to contact us. Veristat has the right resources to help you navigate:
- Clinical Trial Planning
- Clinical Trial Conduct
- Marketing Application Preparation and Post-Market Pharmacovigilance
Adopting Virtual Trial Strategies
Understanding how to navigate the current world situation is made easier by the Veristat COVID team who ensures that all learnings, new virtual trial tools, and regulatory feedback are shared so all sponsors can benefit from shared knowledge.
Our experts support you as you start thinking about bringing your therapy into humans through post-approval.
 Therapeutic Development is Complex
Therapeutic Development is Complex
Veristat understands that nothing is standard in developing medical therapies to save or improve lives. We focus on the most novel, complex, and rare disease therapies. Our teams are passionate and work tirelessly to learn and become experts in each unique disease indication.
We can ensure that your clinical trial or program design supports your regulatory strategy, disease progression analytical models, or previous adult trials. Our experts can help you explore and simulate adaptive trial designs, recruit, and engage the patients and sites through to study completion, and lastly, analyze the data and prepare your submission for regulatory agency review.
To date, of the 760+ development and marketing application projects for rare disease therapies we have worked on:
- Over 60% of the marketing applications we prepared are for rare diseases
- Over 270 of the oncology programs we supported are in rare cancers.
- More than 320 have been rare genetic disease studies
Scientific-Minds Solving Your Critical Problems
Our highly qualified scientific-minded experts aren’t simply executing the plan. They are the strategic thinkers that will evaluate your challenges, create the plan, with multiple options, weighing pros and cons, balancing short- and long-term goals, and identifying the risks involved with every option.
Our clinical development and regulatory submission teams are composed of highly educated experts, among whom more than 65% have advanced degrees. Specifically, our experienced members offer, on average the following industry experience:

