Marketing Applications For Regulatory Approval

Preparing regulatory marketing applications to their successful conclusions is our focus.
Veristat delivers integrated marketing application (NDA, BLA, MAA, NDS, jNDA, etc.) preparation expertise with a track record of success and achievement of seemingly impossible deadlines. Our success lies in our ability to navigate operationally complex submissions, overcome data analysis challenges, and streamline the medical writing process with an integrated team focused on creativity, flexibility, and quality.
Veristat teams supported marketing applications for 8 therapies that received regulatory approval in 2022, the majority for therapies designed to treat rare diseases.
When the Final Milestone is Regulatory Approval
We provide strategic consulting, biostatistical and programming support, CDISC compliant data standardization, and medical writing services to help you prepare and defend your regulatory submissions. We advise on the overall regulatory submission strategy and assist with developing the overall marketing application project plan and timelines. We know how to work collaboratively with other third parties to bring all aspects of the submission timeline together. And, our regulatory publishing team is poised to submit your marketing applications to FDA.
Veristat is proud to have supported the preparation of 80 marketing applications that are now approved for patients. For these approved products, direct involvement included analysis and standardization of the clinical trial data, writing of the application’s modules, and/or publishing to the regulatory agencies.
View our approval record to see how we help sponsors bring life-changing therapies to patients everywhere.
To date, our scientific-minded teams have prepared > 160 Marketing Applications which have led to > 80 Regulatory Approvals from regulatory agencies around the world.
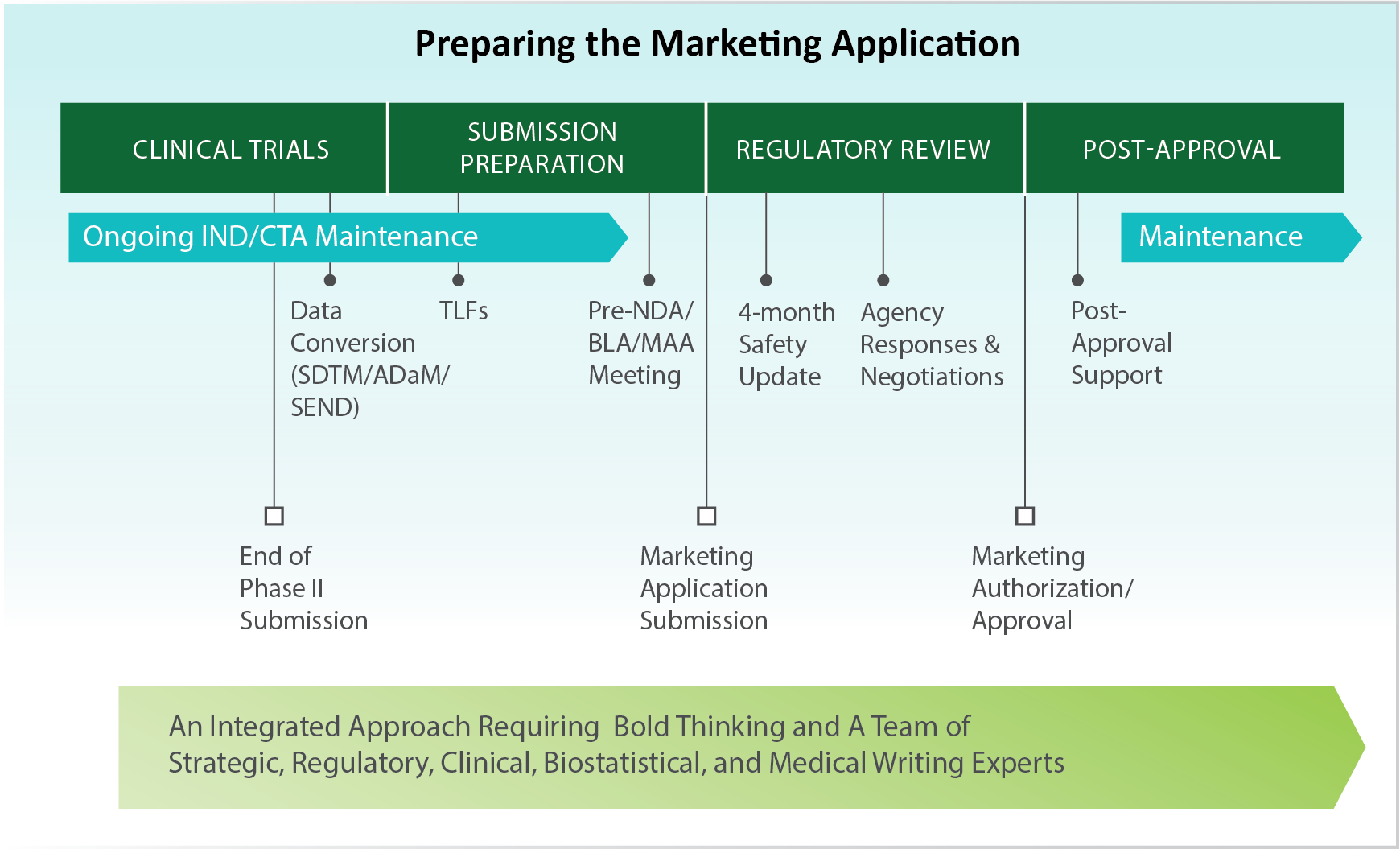
Navigating the Marketing Application Timeline, Preparation, and Publishing Process
Our teams are highly skilled and experienced in supporting the development of the following documents and analyses:
- Phase III Pivotal Clinical Study Reports
- Modules 2.3 and 2.4
- Clinical Overview – Module 2.5
- Summary of Clinical Efficacy (SCE) – Module 2.7.3
- Summary of Clinical Safety (SCS) – Module 2.7.4
![]()
- Module 3 Gap Analysis
- Integrated Summary of Safety (ISS) – Module 5
- Integrated Summary of Efficacy (ISE) – Module 5
- Regulatory Analysis of Overall Application
- SDTM Compliant Dataset
- ADaM Compliant Datasets
- ISS/ISE ADaM Dataset Creation
- Regulatory Briefing documents
- Written and Rapid Responses to Regulatory Authority Questions
- Creation of Slides for Regulatory Presentations
- 4-Month Safety Updates
- Annual Reports
Our Expertise In Marketing Application Preparation
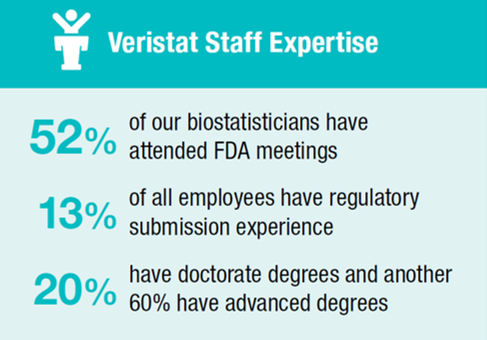 Veristat has been instrumental in the preparation of over 160 marketing applications that have led to 80 approvals so far. Our teams have represented pharmaceutical and biotechnology companies in direct regulatory interaction with the US FDA, Health Canada, European Medicines Agency (EMA), Korea FDA, and Japan FDA.
Veristat has been instrumental in the preparation of over 160 marketing applications that have led to 80 approvals so far. Our teams have represented pharmaceutical and biotechnology companies in direct regulatory interaction with the US FDA, Health Canada, European Medicines Agency (EMA), Korea FDA, and Japan FDA.
We have participated in on-site meetings at regulatory agencies and acted as statistical and clinical experts for advisory panel meetings, including acting as the client statistical representative for many of these panels.
Our senior consultants have over twenty-five years of experience guiding companies through the regulatory submission process. We bring together expert regulatory consultants, biostatisticians, medical writers, data standards professionals, and statistical programmers who work collaboratively to help our clients prepare their regulatory dossiers for submission and continue to support them throughout the agency review process.
Want to ensure a successful regulatory submission process? LET'S TALK