Oncology And Hematology Expertise

When the complexities of designing and executing cancer trials in a highly competitive market can mean delays in delivering urgently needed treatments to patients, it pays to have a trusted partner by your side.
In the past five years, our experienced teams have helped clients with more than 480 oncology and hematology studies, prepared more than 70 INDs/CTAs, and have worked on more than 30 US and European regulatory marketing applications for cancer and hematology treatments.
Our extensive expertise includes traditional cancer treatments, devices/diagnostics as well as specialized therapies including vaccines, gene therapies, cell therapies, and immunotherapies.
- +1000 Oncology & Hematology Projects
- +75 Marketing Applications
- +40 Marketing Application Approvals to Date
Veristat Oncology Center of Excellence
Delivering Results Across a Complex Clinical Development Pathway— Getting You to Market Fast
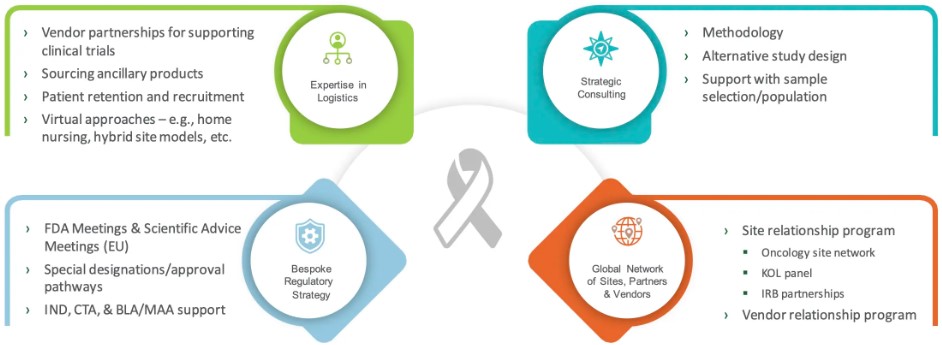 Specialized Oncology CRO from Consultation to Beyond Submission
Specialized Oncology CRO from Consultation to Beyond Submission
Our solutions span the entire clinical development life-cycle - and are offered as a comprehensive, all-inclusive solution or as functional support. We provide strategic guidance for informed decision-making, operational support to mitigate trial risks, the statistical knowledge to prove safety and efficacy, the medical oversight to ensure patient safety, and the regulatory expertise to achieve approval success.
- Pre-Clinical/Trial Planning to Increase Speed
- Study Design and Decentralized Methodologies that Will Work
- Marketing Application Preparation and Publishing
- Post-Market Pharmacovigilance Ensures Safety
"By partnering with a like-minded, scientifically focused company such as Veristat, we have found a core team of high caliber collaborators who can help us to accelerate the development of our new class of targeted cancer therapies."
Chief Medical Officer, MID-SIZE BIOTECH
Oncology & Hematology Highlights
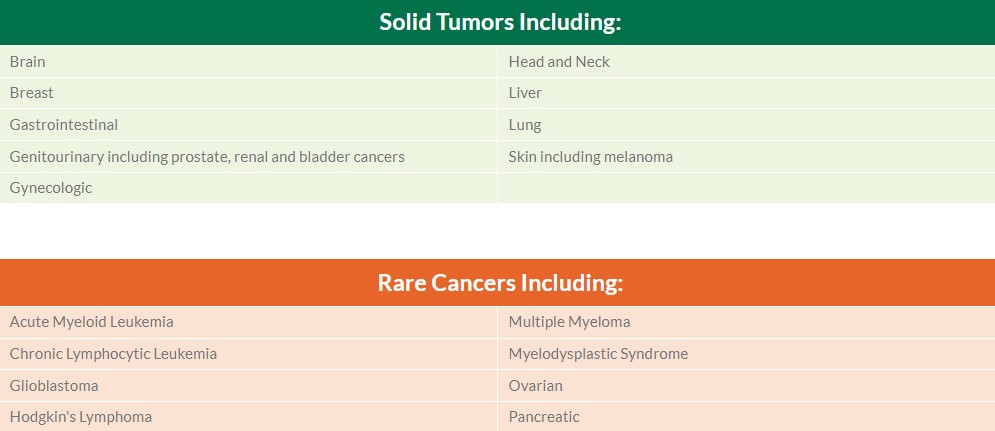
Our expertise spans nearly every solid tumor and hematologic malignancy type, below is a select list of our experience:
US & EU approval for an ultra-rare hematologic malignancy
Regulatory Submission Strategy and Novel Efficacy Endpoint for Treatment of an Ultra-Rare and Aggressive Hematologic Malignancy lead to approval from the FDA and EMA
Focus on Hematology
Veristat has guided clients through the successful conduct of more than 275 hematology projects and has prepared more than 35 marketing applications for blood diseases. Our experience spans clinical development consulting, full clinical trial oversight, and regulatory submission preparation for a broad range non-malignant and malignant hematologic conditions including Leukemias, Lymphomas, and Myelomas.
Some of the rarest hematology indications include complex cell and gene therapeis for diseases such as Beta-Thalasemia, Sickle Cell Disease, Hemophilia and Wiskott-Adrich Syndrome (WAS).
Find out how our clinical research expertise can help with your next study or program.
