Ramblings From A First-Time SCOPE Attendee

By Dan Schell, Chief Editor, Clinical Leader

I am an idiot.
My phone is filled with a bunch of photos of slides that were presented during sessions at the recent SCOPE Summit in Orlando, FL. I’m not sure what I thought I was going to do with all those pictures. It’s not like I can just plop all of them into this show recap and say, “Hey, this was another really cool topic I learned about!” There would be no context, and frankly, I can’t remember why I took some of the photos. (BTW, you can see most of the presentations and slides on the SCOPE app.)
But, did I learn a lot at this conference? Hell yeah! Did I meet some people who I have interviewed before and a lot of new very interesting people who I hope to interview for future articles? Hell yeah! Did I see a giant robot dance on stage during Monday’s introductory remarks and then watch SCOPE Executive Director Micah Lieberman kiss said robot? Uh, yes … yes I did.
Those robot shenanigans weren’t the most memorable part of that early plenary session, though. That award, in my mind, goes to Robert Goodman of Pfizer who, once he took the stage from that shiny opening act, quickly established why he was the headliner of the keynote. In a presentation titled “Time is Life: Pfizer’s Approach to Accelerating Clinical Development,” Goodman mixed patient stories and personal anecdotes with updates on company initiatives. Sure, for the latter, it was interesting hearing about the company’s “Care Everywhere” DCT initiative and PfizerLink research registry. But it was Goodman’s surprising admission that the company’s “Lightspeed” program took its toll on some of the staff that I found most refreshing. After all, you almost never hear from a pharma exec that any program didn’t work out exactly as planned.
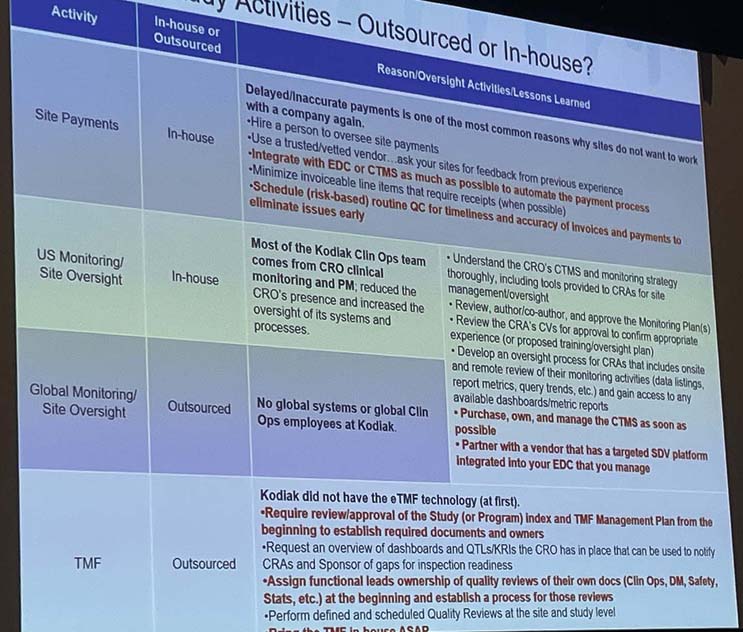
Don’t worry, I’m not going to comment on every session I attended. I will say, though, that there were a few that I really wished were longer. For example, Jamie Christensen, director, at Kodiak Sciences gave a really interesting presentation called, “Challenges and Successes of Bringing Trial Activities In-House While Partnering with a CRO.” Wait! I do have a photo of a slide from her presentation (see below). Although, it’s probably too small to read, it shows different activities like site payments, site monitoring, and TMF and talks about what lessons they learned from executing these activities either in-house or via outsourcing. I loved how she said if you choose to take on some of these activities in-house, “you need to have cash and courage.”
Another topic I would have enjoyed hearing more about was sustainability in clinical trials. In fact, I was surprised by the breadth of issues that fall under the “sustainability” umbrella. During his presentation “Eco Design Tool: Forecasting and Informing Trial Design with a Lens for Environmental Sustainability” Jason Lanier, director, Janssen Clinical Innovation (JCI), talked about the environmental effects of everything from the manufacturing of the drugs themselves (e.g., the energy and resources used to clean vats) to the carbon emissions related to patients traveling back and forth to sites. He even talked about the CO2 emissions from dry ice, which I’m sure everyone could relate to.
The Value Of IRL Networking
Overwhelmingly, everyone I spoke to was impressed with the number of attendees and the breadth of the topics at this year’s SCOPE. One person pointed out that, of course, there were more AI-related sessions than in the past years, and even a few more dedicated to the site perspective. And everyone I spoke with was impressed by the Super Bowl tailgate party!

For me, most of my highlights involved meeting people I’ve interviewed or communicated with over the past few months. I got to chat with Craig Lipset for a few minutes and thanked him for being a long-time editorial board member and just a general supporter of Clinical Leader. I also was pleased to meet Jane Myles, who works with Craig at DTRA (Decentralized Trials & Research Alliance) as program director and who does a fantastic job, among other things, of cohosting the DTRA podcast. Look for an interview with Jane and Craig in future months.
Sticking on the DCT topic, it was great to meet Lindsey Kehoe of CTTI (Clinical Trials Transformation Initiative) in person. She’s one of my three panelists for our upcoming Clinical Leader Live event titled, “Last Year’s DCT is Today’s Clinical Trial.” Lindsey was talking about the current state of DCTs, which is very similar to what we will talk about on that Clinical Leader Live. I also ran into some folks I had just seen two weeks prior at the Save Our Sites (SOS) conference, including Monair McGregor of SiteBridge Research, Jess Thompson of ACRPM (Association of Clinical Research Project Managers), and Brad Hightower of Hightower Clinical. Considering he sat on multiple panels, Brad was almost as visible at SCOPE as he was at SOS.
Overall, my opinion: SCOPE was great. Hats off to Micah Lieberman and all the staff at Cambridge Innovation Institute for producing such an informative and well-organized event. I look forward to attending next year — with or without that giant robot.

